Everything that has mass and occupies space volume














- Slides: 33

• Everything that has mass and occupies space (volume) is called matter. All matter has some General Properties. • Matter is the “amount of stuff” in an object. – The mass of an object is a measure of the amount of matter the object contains. – The volume of an object is a measure of the space occupied by the matter. – The density of an object is a measure of its mass / volume

KINETIC (MOLECULAR) THEORY • The kinetic molecular theory attempts to explain how microscopic particles in matter behave. • It states that all matter (solids, liquids, gases) are comprised of atoms, ions, molecules or compounds that are in constant motion. The basis for this motion is Kinetic Energy.

KINETIC (MOLECULAR) THEORY The Kinetic Molecular Theory has several assumptions: 1. All matter is comprised of small subatomic particles (atoms, molecules, compounds 2. Particles constantly move very fast in random motion. 3. These particles travel in straight line and only collide with one another and their container.

• Kinetic energy is the energy associated with the motion of objects (large or small objects). • Temperature is a measure of the average kinetic energy (motion) of a material. • Higher Temp, Higher KE (Kinetic Energy)

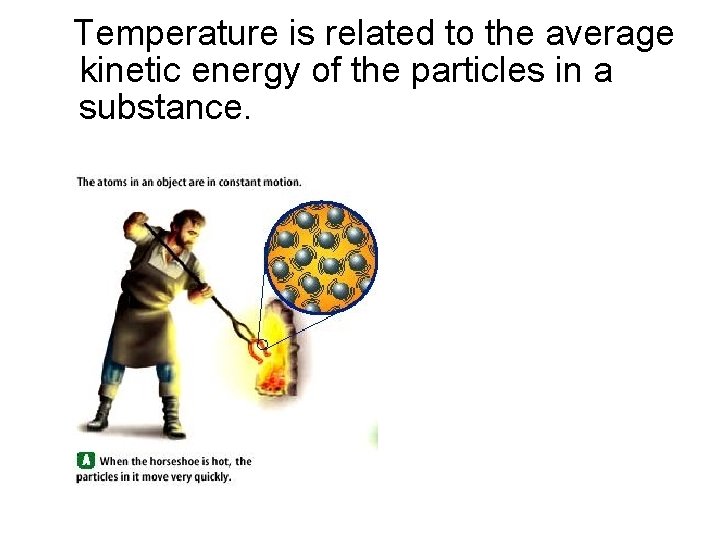
Temperature is related to the average kinetic energy of the particles in a substance.

• As a solid, liquid or gas is heated, the average kinetic energy (speed) of the molecules increases and the materials volume (space) increases. • The increase in the volume of a substance is called Thermal Expansion. • Thermal expansion dependents on: 1. The type of material (matter). 2. The temperature of the material.

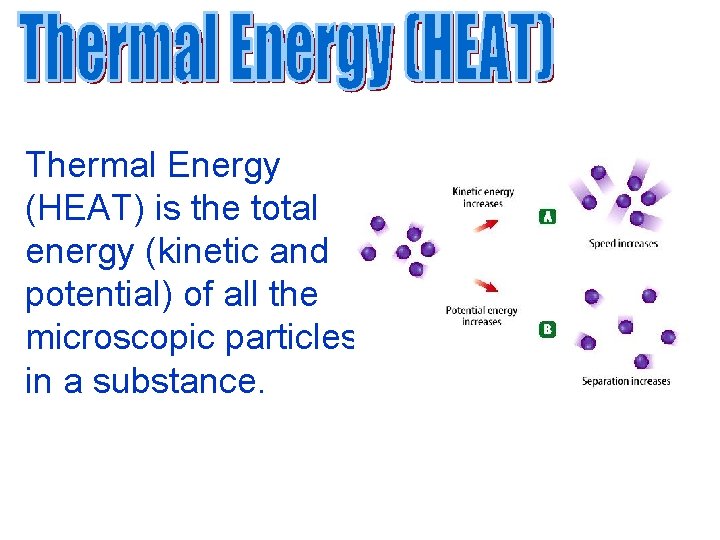
Thermal Energy (HEAT) is the total energy (kinetic and potential) of all the microscopic particles in a substance.

• Solids • Liquids • Gases

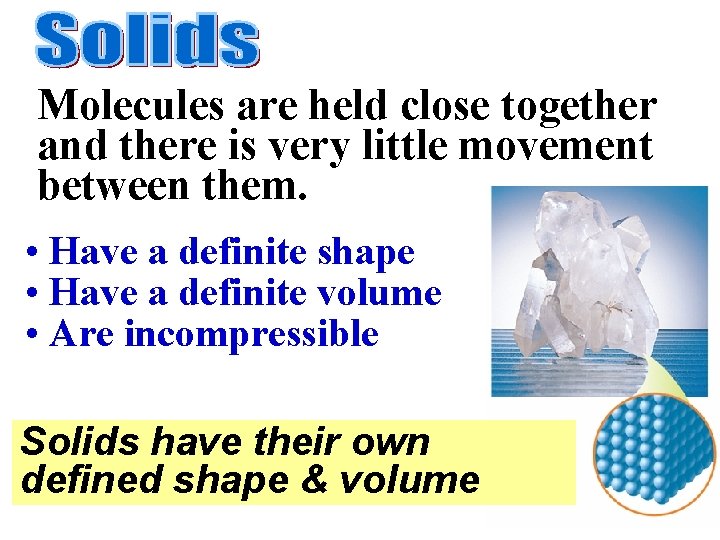
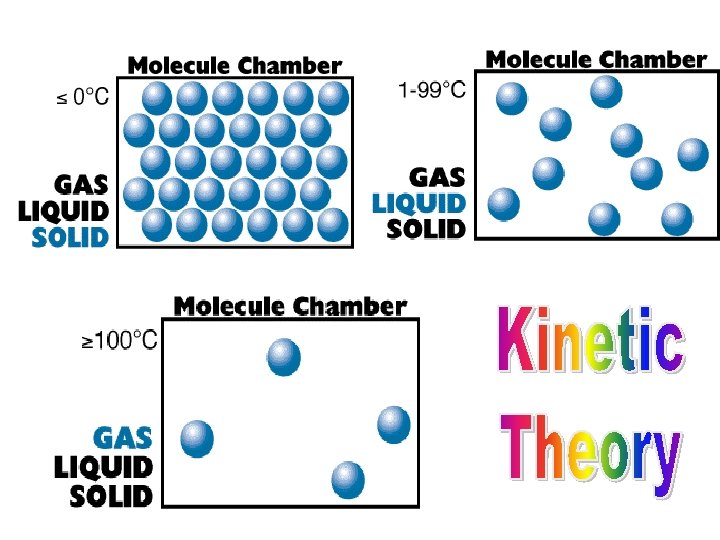
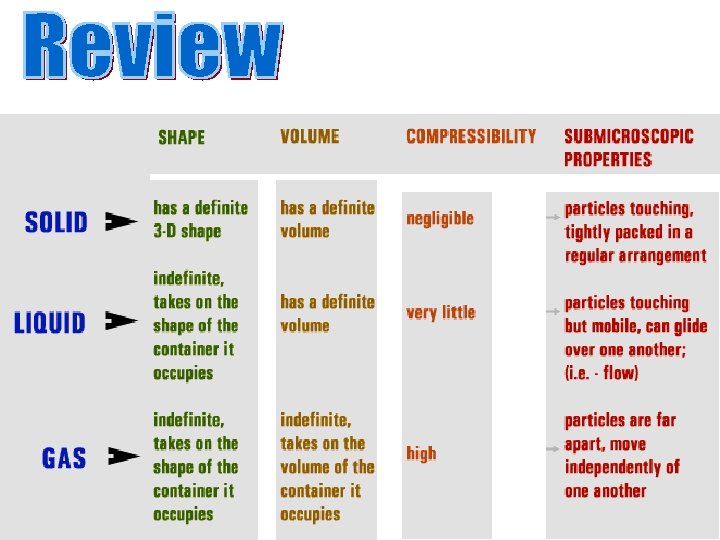
Molecules are held close together and there is very little movement between them. • Have a definite shape • Have a definite volume • Are incompressible Solids have their own defined shape & volume

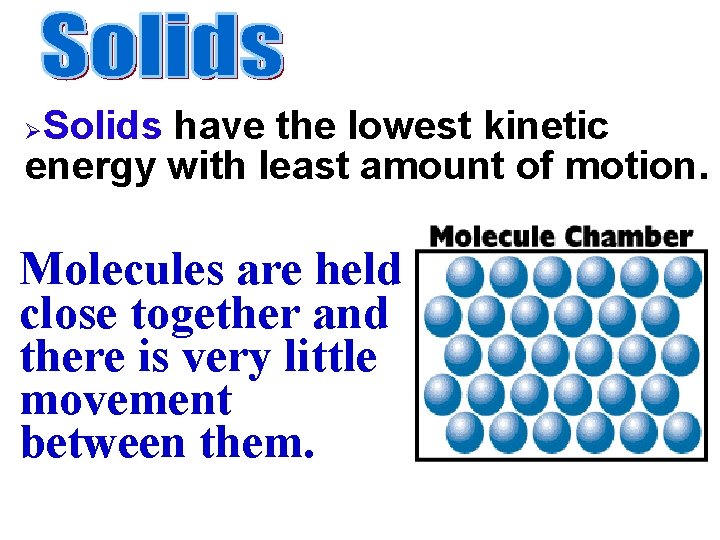
Solids have the lowest kinetic energy with least amount of motion. Ø Molecules are held close together and there is very little movement between them.

Liquids have the next greatest kinetic energy with the next highest motion. Ø Atoms and molecules have more space between them than a solid does, but less than a gas (ie. It is more “fluid”. )

• Have an indefinite shape • Have a definite volume • Are not easily compressed Liquids conform to the shape of their container and have a defined volume

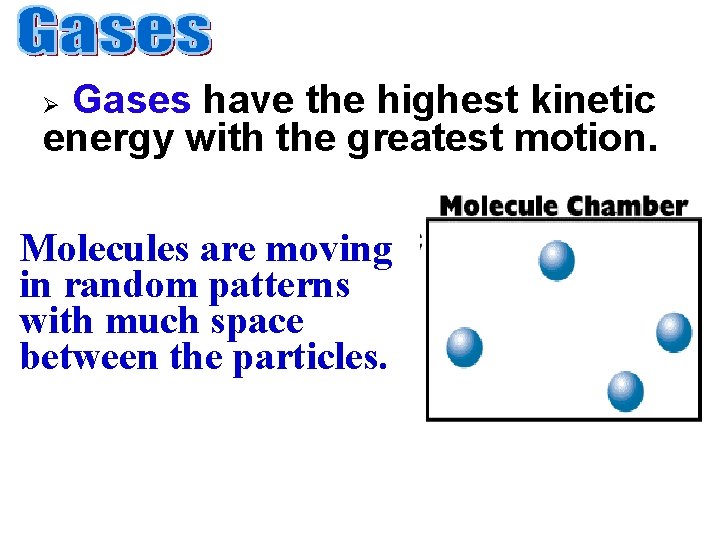
Gases have the highest kinetic energy with the greatest motion. Ø Molecules are moving in random patterns with much space between the particles.

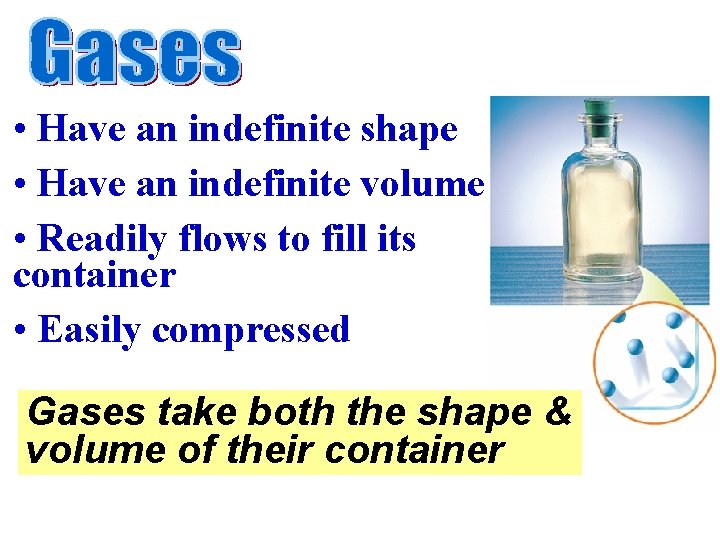
• Have an indefinite shape • Have an indefinite volume • Readily flows to fill its container • Easily compressed Gases take both the shape & volume of their container


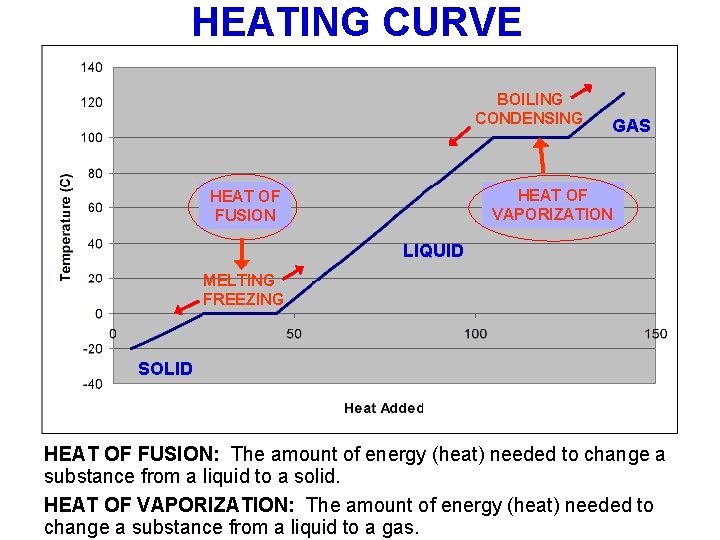
HEATING CURVE BOILING CONDENSING GAS HEAT OF VAPORIZATION HEAT OF FUSION LIQUID MELTING FREEZING SOLID HEAT OF FUSION: The amount of energy (heat) needed to change a substance from a liquid to a solid. HEAT OF VAPORIZATION: The amount of energy (heat) needed to change a substance from a liquid to a gas.

State of matter – 1 min

• Matter that exists in a gaseous state at room temperature is called a “gas”. • “Vapor” is the gaseous state of a substance that is a liquid at room conditions. • So air is composed of nitrogen and oxygen gases and water vapor.


3 min

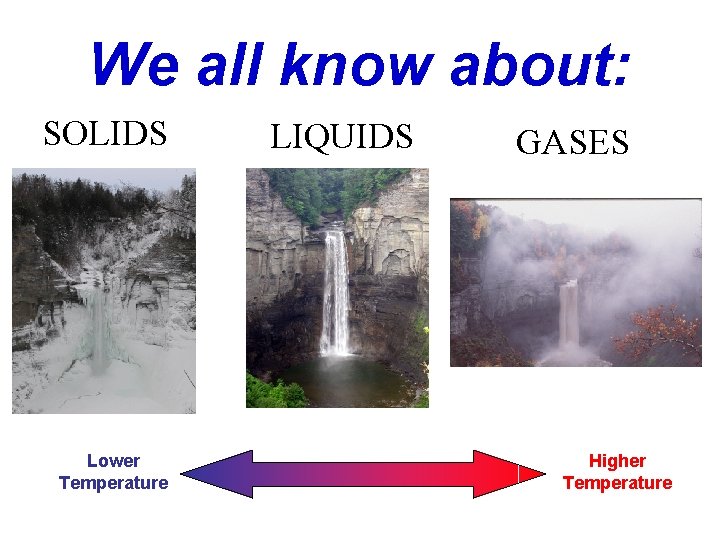
We all know about: SOLIDS Lower Temperature LIQUIDS GASES Higher Temperature

But what happens if you raise the temperature to super-high levels… between 1000°C and 1, 000, 000°C ? Will everything just be a gas?

NO! If the gas: – is made up of particles which carry an electric charge (“ionized particles”) and electrons, – and the entire gas as a whole has no electric charge, – and if the density is not too high, Then we get the 4 th state of matter: PLASMA

• Plasma is by far the most common form of matter. • Plasma in the stars and in the tenuous space between them makes up over 99% of the visible universe and perhaps most of that which is not visible.

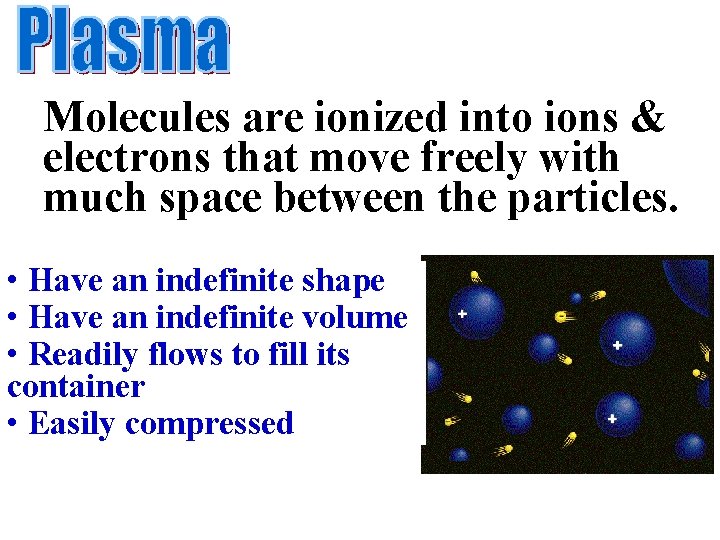
Molecules are ionized into ions & electrons that move freely with much space between the particles. • Have an indefinite shape • Have an indefinite volume • Readily flows to fill its container • Easily compressed

So now we know all about four states of matter: SOLIDS Lower Temperature LIQUIDS GASES PLASMAS (only for low density ionized gases) Higher Temperature

But now what happens if you lower the temperature way, down to 100 nano degrees above “Absolute Zero” (-273°C) Will everything just be a frozen solid?

Not Necessarily! In 1924 (87 years ago), two scientists, Albert Einstein and Satyendra Bose predicted a 5 th state of matter which would occur at very low temperatures. Einstein Bose +

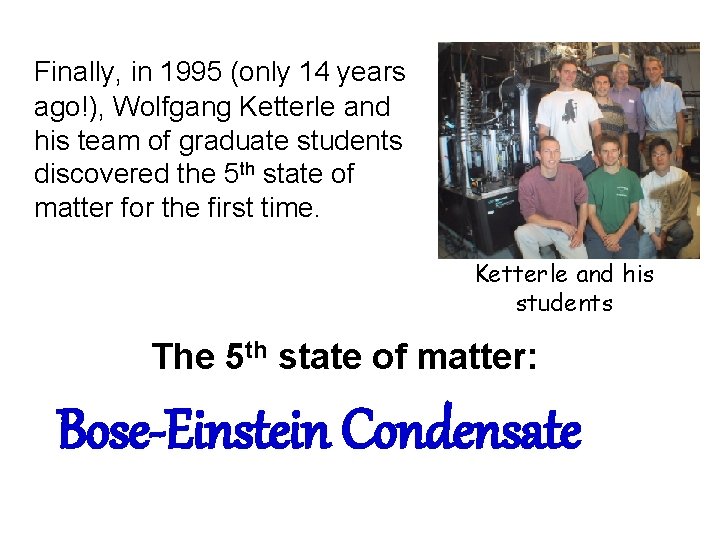
Finally, in 1995 (only 14 years ago!), Wolfgang Ketterle and his team of graduate students discovered the 5 th state of matter for the first time. Ketterle and his students The 5 th state of matter: Bose-Einstein Condensate

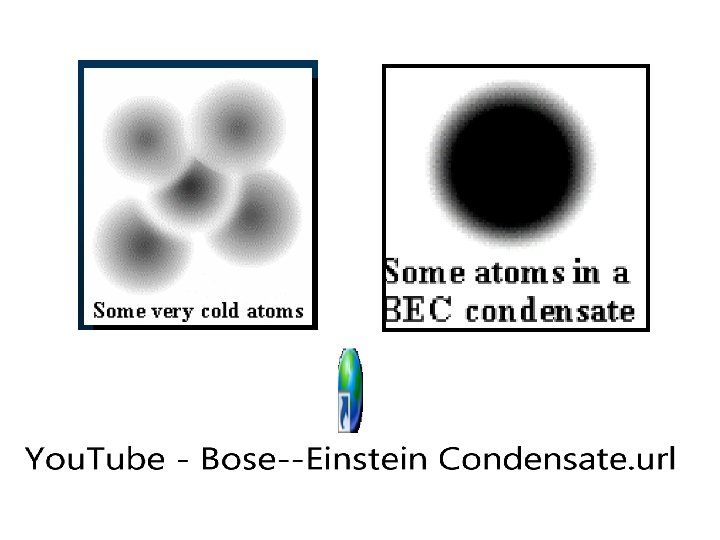
In a Bose-Einstein condensate, atoms can no longer bounce around as individuals. Instead they must all act in exactly the same way, and you can no longer tell them apart!


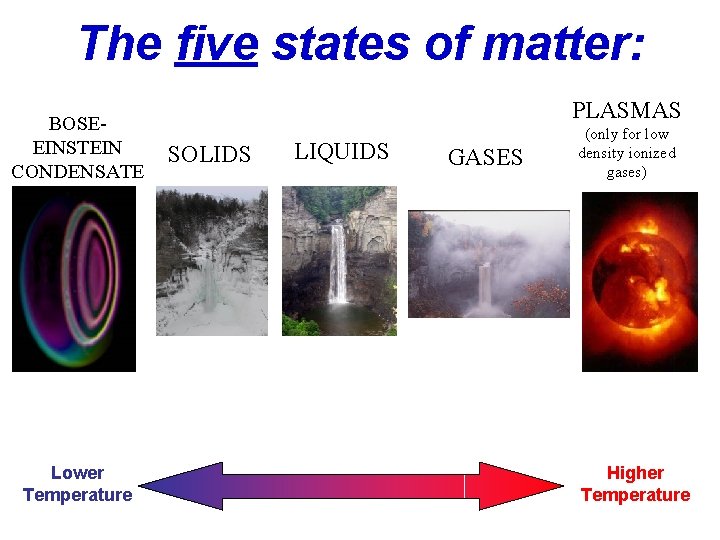
The five states of matter: BOSEEINSTEIN CONDENSATE Lower Temperature PLASMAS SOLIDS LIQUIDS GASES (only for low density ionized gases) Higher Temperature

How would you do the following? • How would you separate a mixture of the following materials in one big beaker? • Sand • Salt • Water • Small pebbles • Iron filings • Sawdust