Ever wonder why Episode 901 Kinetic Theory Gases










- Slides: 14

Ever wonder why? Episode 901

Kinetic Theory • Gases are composed of small, separate particles called molecules. • Gas molecules are in constant motion. • All collisions between particles are perfectly elastic. • The molecules of a gas display no attraction or repulsion for one another. • The average kinetic energy of the molecules is directly proportional to the Kelvin temperature of the gas. Episode 901

Ideal Gas • Gas whose behavior conforms to the kinetic theory – it is theoretical. • All gases behave as an ideal gas unless temperature is very low or pressure is very high. Episode 901

Gas Pressure • Pressure = force / area • Atmospheric Pressure – the pressure the earth’s atmosphere exerts due to its weight Episode 901

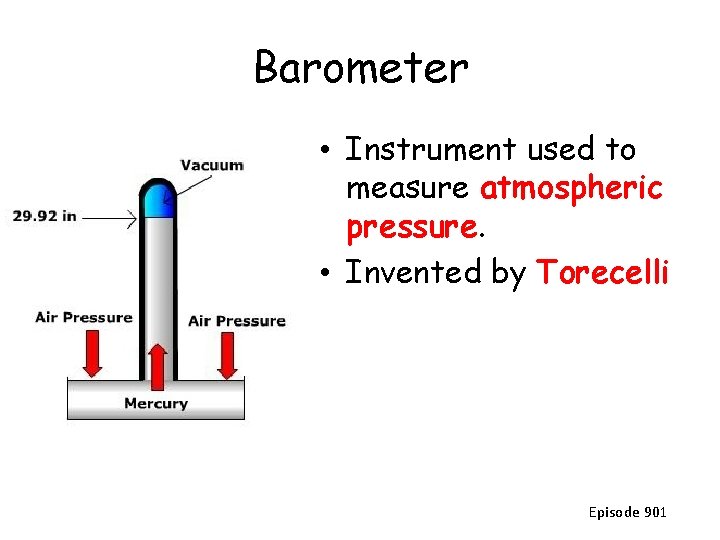
Barometer • Instrument used to measure atmospheric pressure. • Invented by Torecelli Episode 901

Normal Atmospheric Pressure • • • Also called standard pressure 760 mm Hg 760 torr 1 atm (atmosphere) 101. 3 k. Pa STP • Standard Temperature and Pressure • 1 atm • 0⁰Celsius Episode 901

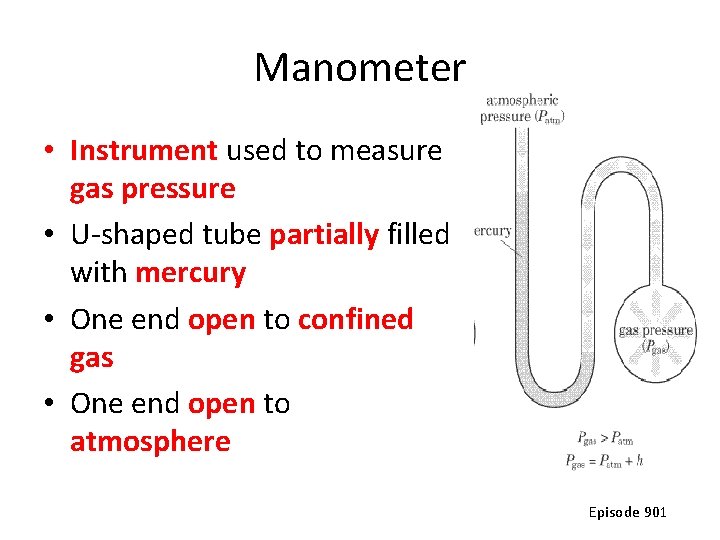
Manometer • Instrument used to measure gas pressure • U-shaped tube partially filled with mercury • One end open to confined gas • One end open to atmosphere Episode 901

CR Question 1 • What would be the density of a 10 gram object that displaces two milliliters of water? a. Five b. 20 c. Five grams per milliliter d. 20 grams per milliliter Episode 901

CR Question 2 • Is oxygen a metal, nonmetal, or metalloid? a. Metal b. Nonmetal c. Metalloid Episode 901

Review Question 1 • Does the average kinetic energy of the gas particles in an inflated inner tube increase or decrease if the sun heats the inner tube from 20 degrees Celsius to 30 degrees Celsius? a. Increase b. Decrease Episode 901

Review Question 2 • What is the name of the instrument used to measure the pressure of a confined gas? a. Manometer b. Pascalometer c. Pressureometer d. Barometer Episode 901

Review Question 3 • STP is a. Standard temperature and pressure b. Zero degrees Celsius and one atmosphere pressure c. Zero degrees Celsius and one hundrend one point three kilopascals d. All of these Episode 901

Review Question 4 • Under what conditions of temperature and pressure does the behavior of a real gas differ from that of an ideal gas? a. Very high temperature and very high pressure b. Very high temperature anf very low pressure c. Very low temperature and very low pressure d. Very low temperature and very high pressure Episode 901

Review Question 5 • The kinetic theory a. Is a model b. Explains gas behavior c. Neither a nor b d. Both a and b Episode 901
 Torecelli
Torecelli Kenetic particle theory
Kenetic particle theory Have you ever wondered questions
Have you ever wondered questions Kinetic molecular theory
Kinetic molecular theory Kinetic theory of gases
Kinetic theory of gases Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic theory
Kinetic theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases General gas equation is
General gas equation is Did you ever wonder
Did you ever wonder Pictures
Pictures Ever ancient ever new
Ever ancient ever new Ever ancient ever new
Ever ancient ever new Ever ancient ever new
Ever ancient ever new



























