Even More About Water Lesson 2 part 2

Even More About Water Lesson 2 part 2

Mixtures • Heterogeneous Mixtures • Parts of the mixture are noticeably different from one another • Homogenous Mixtures • Evenly distributed, hard to tell the difference between one part and another

Solutions • Type of homogenous mixture • When substances dissolve in another • Particles are too small to settle out, filtered or scatter light

Solvent vs Solute • Water has many properties, but one of the most important is that water acts as a UNIVERSAL solvent • Solvent – Substance that does the dissolving • Solute – Substance that dissolves in the solvent • The solubility of a substance is its ability to dissolve another substance • Water is very good at dissolving • Hot water can hold more solute (can dissolve more) than cold water

Water: The Universal Solvent • Water’s polarity contribute to water as a universal solvent • Salt is part metal, part nonmetal • Metal and nonmetal bonding form ionic bonds • Ionic bonds form from IONS • Elements that have a + or – charge • Opposites Attract

Ocean • Ocean is filled with dissolved particles • Elements that have opposite electrical charges are called SALTS • This makes a Saline solution • Na, Cl, Sulfate, Magnesium, Calcium, Potassium, Bicarbonate • Distilled water = no salinity • Fresh water is. 01% salt or less • Ocean average is 3. 5% salt or more • Every liter has 1. 2 ounces of salt

Why is the Ocean Salty? • Water’s excellent ability to dissolve solutes means it is carrying materials with it • Water does the same in all organisms • Where do these salts come from? • Fresh water & streams contain salts • Rivers dump into the oceans • 4 billion tons of salts are emptied into the oceans each year • Water also evaporates • Leaves behind salts

Where else? • Inside the Earth • Deep Sea Vents • From the Atmosphere • Wind and Rain carry minerals • Human made • Only removed by incorporating into rocks, sediments and organisms

Salinity & Marine Organisms • Marine organisms are designed to live in the salt water • Brackish = in between salty or fresh • Mangrove forests and salt marshes • Called estuaries • Where rivers meet oceans • Many juvenile organisms live here • “Nursery of the Sea” • Also protect coastlines from erosion
Regulation of Solute/Water Balance • Marine organisms go through Osmosis • Water moves from low salinity (high water concentration) to high salinity (lower water concentration • Osmoconformers • Do not attempt to control solute/water balance • Their internal concentration varies as the salinity in the water around them changes • Most can only tolerate a very narrow range of salinity
Regulation of Solute/Water Balance • Osmoregulators – These organisms control their internal concentrations – Can generally tolerate a wider range of salinities than osmoconformers – Some animals require different amounts of salt for different lifecycles – This can be done in a variety of ways such as secreting very little urine or using specialized glands to secrete salts as examples

Salt & Organisms • Osmoregulation – Individual cells control the balance of water • Mangroves will release salt from their leaves • Some sea turtles and marine birds have special salt glands that remove excess salt
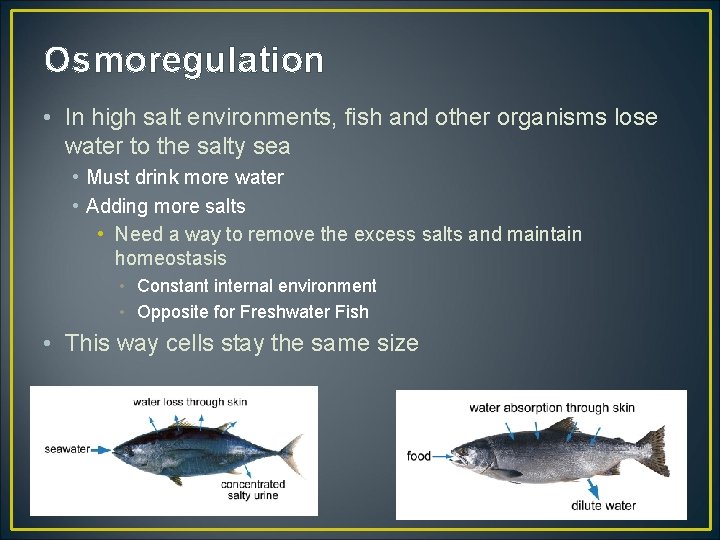
Osmoregulation • In high salt environments, fish and other organisms lose water to the salty sea • Must drink more water • Adding more salts • Need a way to remove the excess salts and maintain homeostasis • Constant internal environment • Opposite for Freshwater Fish • This way cells stay the same size
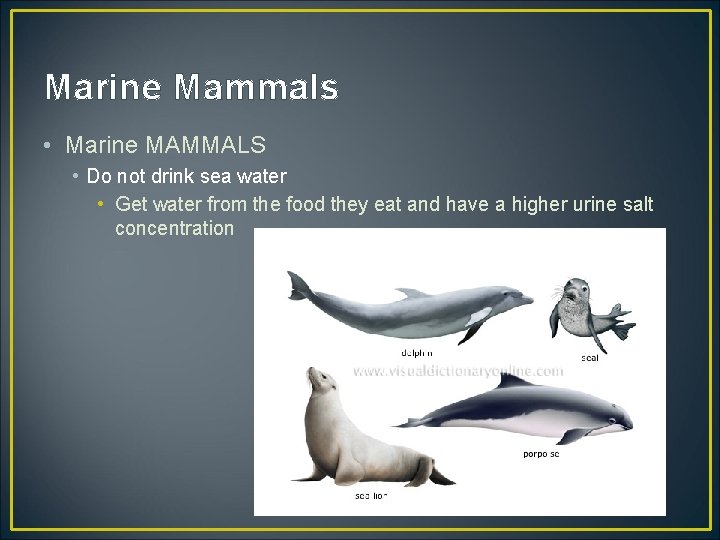
Marine Mammals • Marine MAMMALS • Do not drink sea water • Get water from the food they eat and have a higher urine salt concentration

Density and Marine Organisms • Maintain neutral buoyancy • Float, sink Less dense than water
- Slides: 15