EVALUATION OF THE CARBONIC ACID EQUILIBRIUM Ukrainian State

EVALUATION OF THE CARBONIC ACID EQUILIBRIUM Ukrainian State University of Chemical Technology, Ukraine

Lab purpose § Task • To study the alkalinity and buffering capacity of several types of water: surface water, groundwater (mineral water), and sea water § Objectives • To observe shifting of carbonic acid content vs. the source of the water sample • To get experience to calculate the alkalinity in different waters • To determine the buffering capacity in selected water samples by the titration method CARBONIC ACID EQUILIBRIUM 2

List of equipment § § § § Calibrated p. H meter (with titration sell if available, like shown on fig. 4) Stirring plate Micro magnetic stir bar Clean polyethylene bottles Burette with burette stand porcelain tittle Volumetric pipets with elongated tips Pipette bulb, or pipette pump Syringe equipped with a filter holder and a fine-pore filter Conical flask (Erlenmeyer flask) 250 ml measuring syringe Standard flask Wash bottle Beakers CARBONIC ACID EQUILIBRIUM 3
Theory Of all acid-base systems, the most important is the carbonic acid system comprising CO 2, HCO 3 -, and CO 32 -. These components govern many different processes, from conversions in the global carbon cycle to the p. H balance of blood, and, of course, the properties alkalinity and hardness of natural waters. For water transported in pipes or stored in tanks the probability of corrosion reactions with the material of the pipe and the tank is strongly dependent on its carbonic acid content. Therefore it is very important to measure and control the different carbonic acid components in water samples. CARBONIC ACID EQUILIBRIUM 4
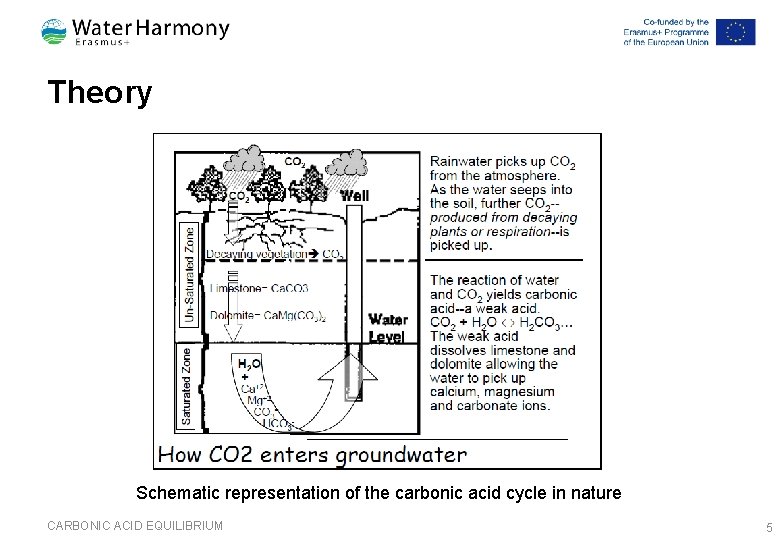
Theory Schematic representation of the carbonic acid cycle in nature CARBONIC ACID EQUILIBRIUM 5
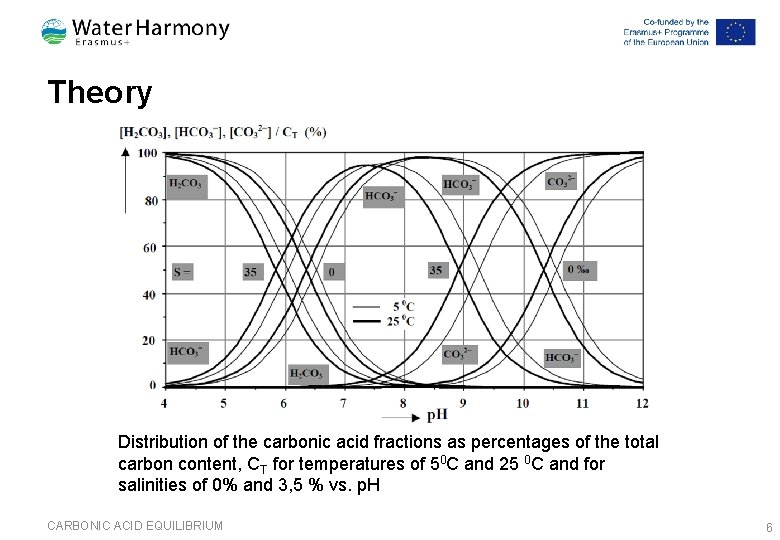
Theory Distribution of the carbonic acid fractions as percentages of the total carbon content, CT for temperatures of 50 C and 25 0 C and for salinities of 0% and 3, 5 % vs. p. H CARBONIC ACID EQUILIBRIUM 6
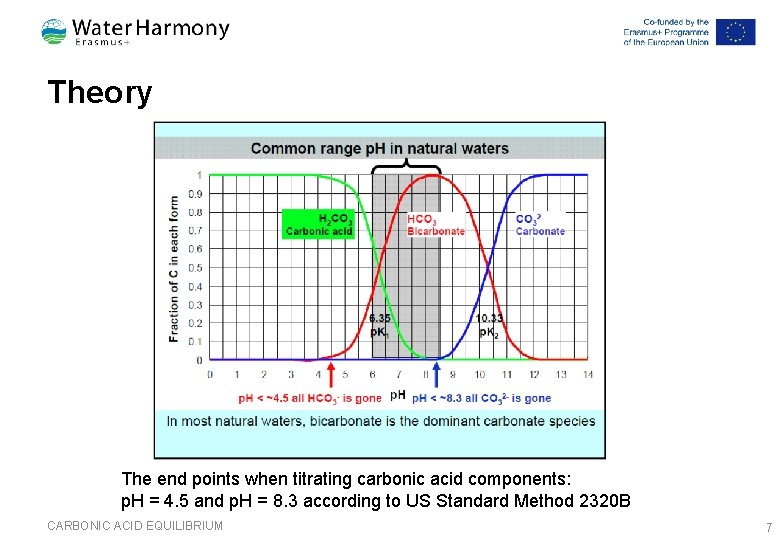
Theory The end points when titrating carbonic acid components: p. H = 4. 5 and p. H = 8. 3 according to US Standard Method 2320 B CARBONIC ACID EQUILIBRIUM 7
Tasks performance order § Experiment 1: Sampling and titration § Experiment 2: Evaluation of the titration curves and endpoint analysis CARBONIC ACID EQUILIBRIUM 8

Report content Please replace „Experimental Title“ by „Water Sample“ CARBONIC ACID EQUILIBRIUM 9

Report content CARBONIC ACID EQUILIBRIUM 10
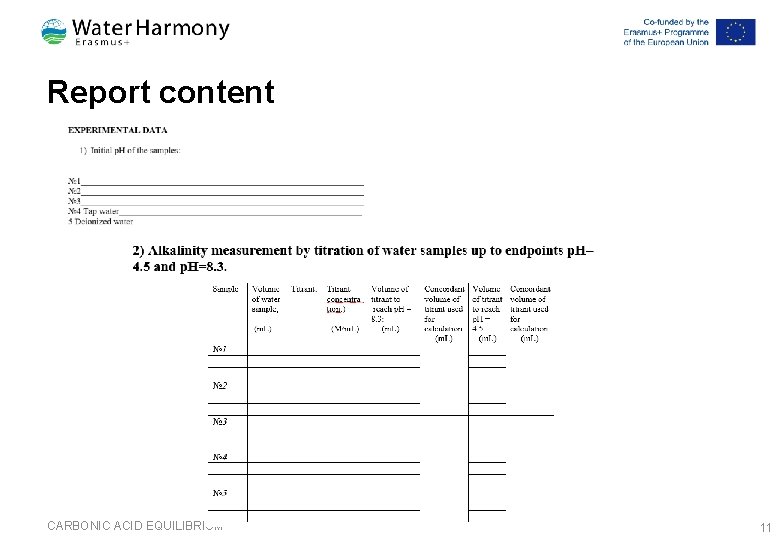
Report content CARBONIC ACID EQUILIBRIUM 11
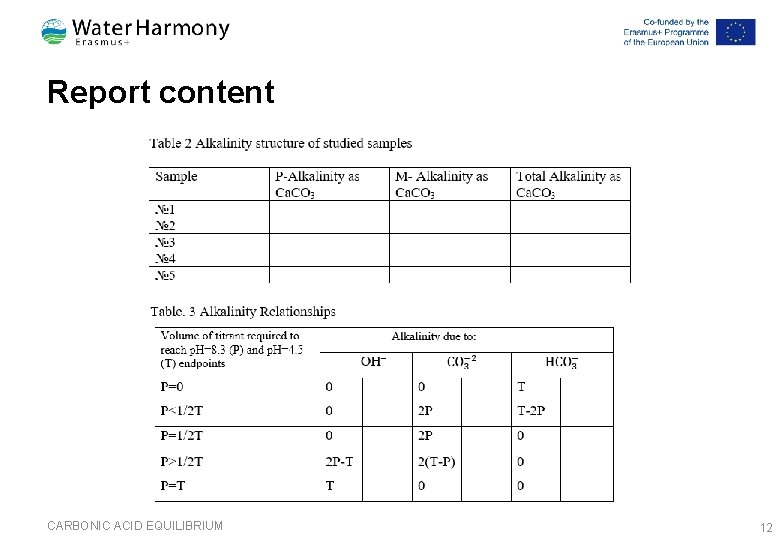
Report content CARBONIC ACID EQUILIBRIUM 12
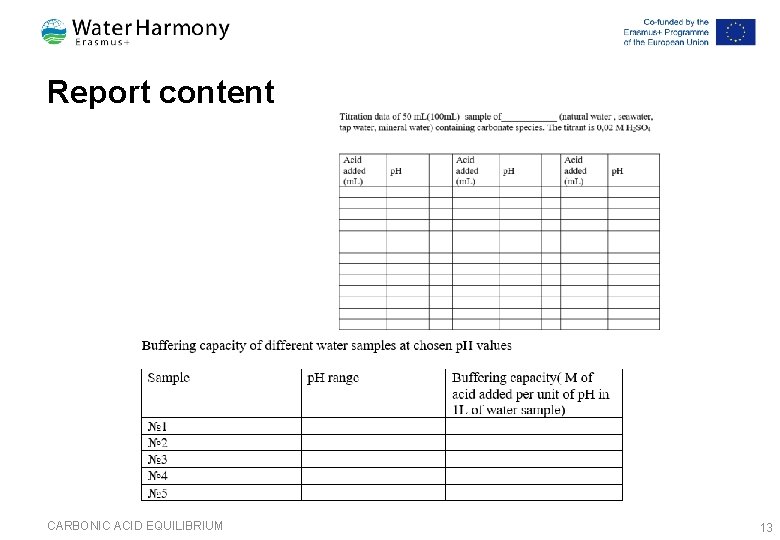
Report content CARBONIC ACID EQUILIBRIUM 13

Final discussion § Why is distilled water or DI water considered to have almost no alkalinity and buffering capacity? § Why does the sample of seawater exhibit a high alkalinity and buffering capacity? § What is the reason for the buffering capacity observed in surface waters? CARBONIC ACID EQUILIBRIUM 14

Recommended literature § APHA Standard methods for the examination of water and wastewater 20 th edition: Method 2320. § Methods for chemical analysis of water and wastes EPA 600/4 -79 -020, USEPA: Method 310/1. § Dickson, A. G. , C. L. Sabine and J. R. Christian (2007): Guide to best practices for ocean CO 2 measurements, pp 191. § Keeling, C. D. and T. P. Whorf (2006): Atmospheric CO 2 records from sites in the air sampling network, in: Trends - A Compendium of Data on Global Change. CDIAC, Oak Ridge National Laboratory, U. S. Department of Energy, Oak Ridge, Tenn. , USA § Millero, F. J. , F. Huang, T. Graham and D. Pierrot (2007): The dissociation of carbonic acid in Na. Cl solutions as a function of concentration and temperature, Geochim. Cosmochim. Acta 71, 46 -55. § Andersen. C. B. (2002): Understanding Carbonate Equilibria by Measuring Alkalinity in Experimental and Natural Systems, Geochem. Educ. 50, 389 -403. § Dunnivant, F. M. (2004): Experiment 21 (Determination of Alkalinity of Natural Waters), in: Environmental Laboratory Exercises for Instrumental Analysis and Environmental Chemistry, Wiley-Interscience, New York, USA. CARBONIC ACID EQUILIBRIUM 15
- Slides: 15