Evaluation of Sampling Containment Glass vs Low Density



- Slides: 12

Evaluation of Sampling Containment Glass vs Low Density Plastic 2017 Multi-Agency Harmful Algal Bloom Symposium January 18, 2017 Presented by: Tony Stahl and Layne Knight, KDHE Our Mission: To protect and improve the health and environment of all Kansans.

Effect of Storage Container on Toxin Recovery

Sample Collection Container Assessed • Clear or amber glass • High density polyethylene (HDPE) • Polyethylene terephthalate glycol (PETG) • Polycarbonate (PC) • Polypropylene (PP) • Polystyrene (PS)

Recommend Sample Collection Container Collection and storage materials included glass and various types of plastics. It was found that microcystin congeners LA and LF adsorbed to polystyrene, polypropylene, high density polyethylene and polycarbonate storage containers, leading to low recoveries (<70%), cylindrospermopsin and saxitoxin did not adsorb to the containers tested (Kamp et al. , 2016). Abraxis recommends for Microcystins • Clear or amber glass • Polyethylene terephthalate glycol (PETG) • Avoid all plastic containers other than PETG, as toxin will be lost due to adsorption to container surface.

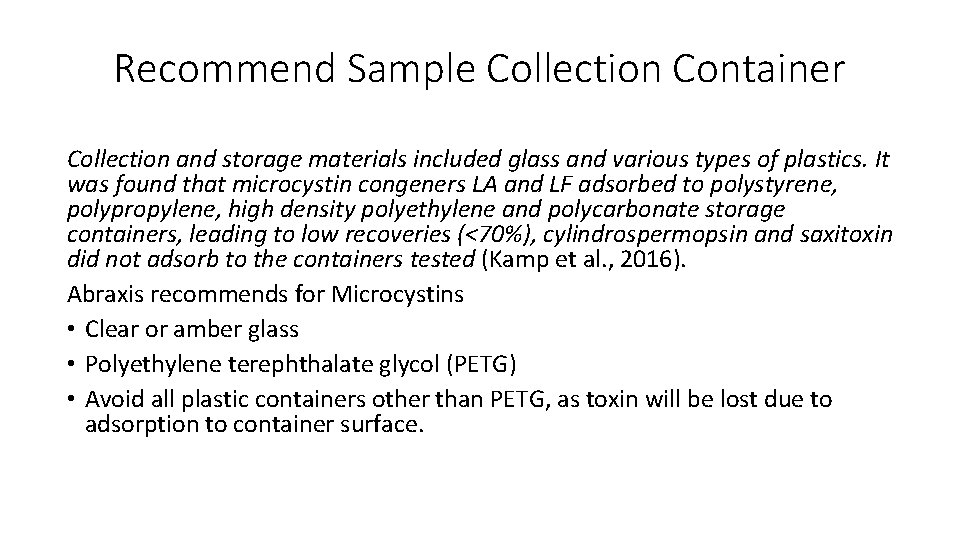
Evaluation of Sampling Containment • Glass vs Plastic (LDP/LLDP) • Cubitainers made of low density polyethylene (LDP) and Low linear density polyethylene (LLDP) were evaluated for their efficiency in extracting toxin compared to amber glass sample containment. • The cubitainers are extremely durable and maintain containment integrity during transport or shipment.

Evaluation of Sampling Containment Analysis: Abraxis microcystin ELISA immunoassay following the Ohio EPA ELISA-ADDA analytical standard operating procedure. • Working Range: 0. 3 to 5. 0 µg/L • Reporting Limit: 0. 3 µg/L (or ppb) Collection: Raw water samples were collected in replicate (in glass and low density plastic ). After shaking and pouring ¾ out, all samples underwent two freeze/thaw lysing cycles.

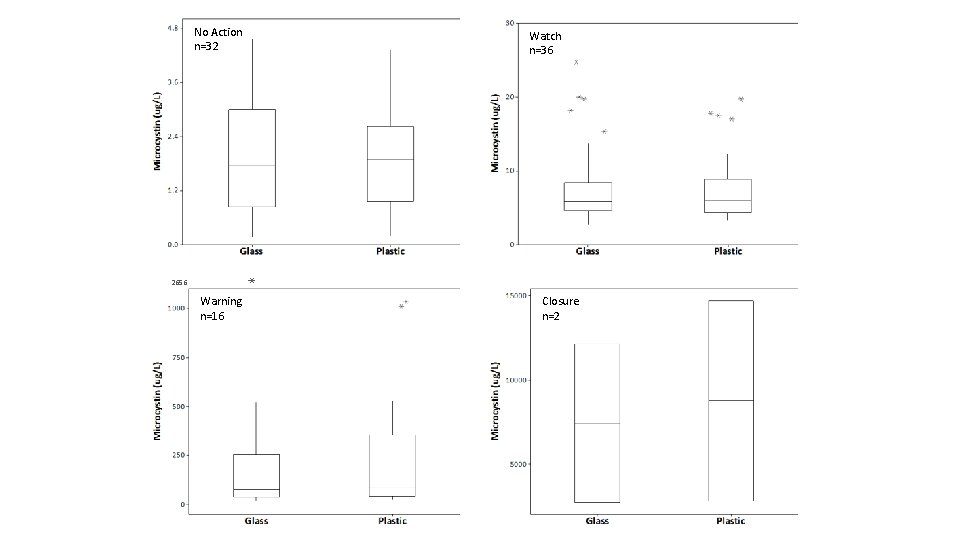
Results • Full Dataset (n=88) • Two pairs of analyses excluded (e. g. glass = 3. 712 μg/L vs. plastic = 0. 008 μg/L) • Log 10 transformed to achieve normality • Conducted graphical examination and Paired t-test • Trimmed Dataset (n=86) • • No Action (<4. 0 μg/L) n=32 Watch (4. 0 – <20. 0 μg/L) n=36 Warning (20. 0 – 2000 μg/L) n=16 Closure (>2000 μg/L) n=2

No Action n=32 Watch n=36 2656 Warning n=16 Closure n=2

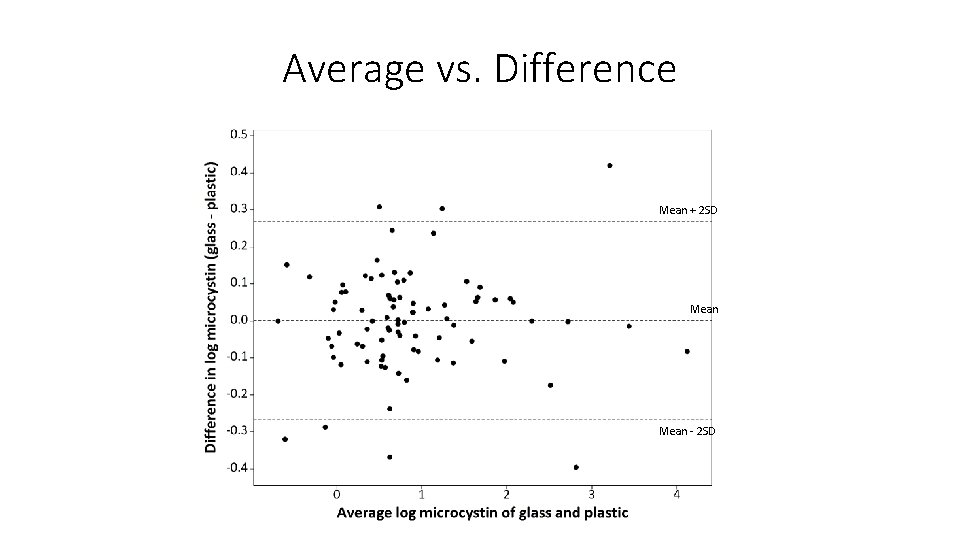
Average vs. Difference Mean + 2 SD Mean - 2 SD

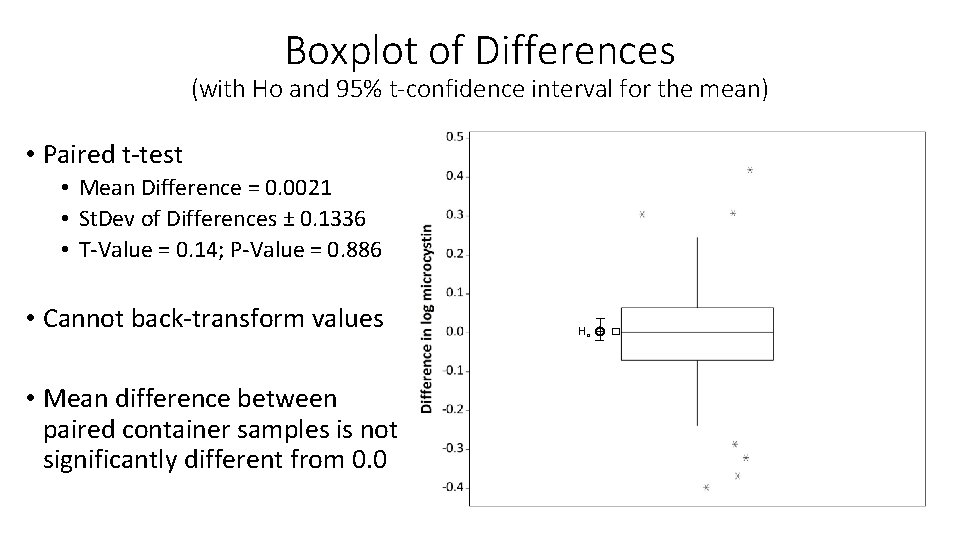
Boxplot of Differences (with Ho and 95% t-confidence interval for the mean) • Paired t-test • Mean Difference = 0. 0021 • St. Dev of Differences ± 0. 1336 • T-Value = 0. 14; P-Value = 0. 886 • Cannot back-transform values • Mean difference between paired container samples is not significantly different from 0. 0 Ho �

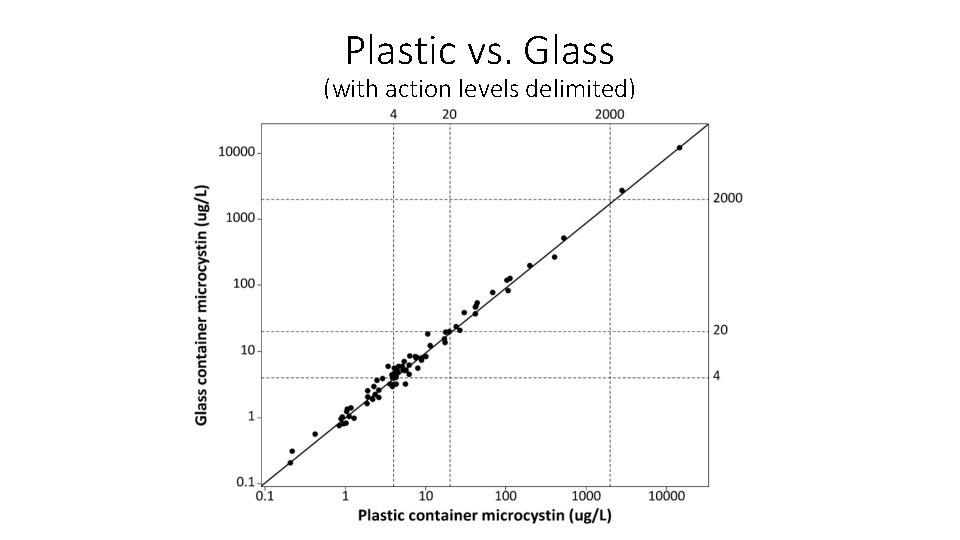
Plastic vs. Glass (with action levels delimited)

Other Products and Future Direction • Major boost to cell count vs. cyanotoxin database (n = 1389) • Greater resolution at low toxin levels • Confirmation of Envirologix qualitube based toxicity values • Repeatability of Abraxis microtiter plate technique • Multiple analyses of same sample** • Toxin loss analysis using microcystin known standards (fortified samples of microcystin)