Evaluation and Management of Fabry Disease Supported by

Evaluation and Management of Fabry Disease Supported by Sanofi Genzyme

Agenda • Pathogenesis of Fabry Disease • Evaluation of Fabry Disease • Fabry Disease Management

Learning Objectives • Discuss the pathophysiology of Fabry disease and its impact on kidney function to increase awareness • Describe appropriate clinical diagnosis in Fabry disease, and explain the role of nephrologists in the early identification of patients • Describe high risk patients as target of screening, including hereditary aspect of Fabry disease • Discuss methods of testing for Fabry disease and those currently available • Discuss treatment strategies in kidney disease patients with Fabry disease, including symptom management and addressing the underlying pathology, to improve patient outcomes

Pathogenesis of Fabry Disease

Fabry Disease • An X-linked lipid storage disorder • Deficient or absent lysosomal α-galactosidase A (α-gal A) activity systemic deposition of glycosphingolipids [mainly globotriaosylceramide (Gb 3 or GL 3)] • Affects the heart, kidney, and neurologic systems, but can impact all organs • Is considered a genetic risk factor for kidney disease, cardiomyopathy, stroke, and early death
Epidemiology • Fabry disease is pan-ethnic, but due to its rarity, determining an accurate disease frequency is difficult • Reported incidence: 1/47, 600 to 1/117, 000 in the general population, but the true prevalence is probably much higher • Newborn screening: – 1/~3, 100 newborns in Italy – 1/~1, 500 newborns in Taiwan (86% with cardiac variant)
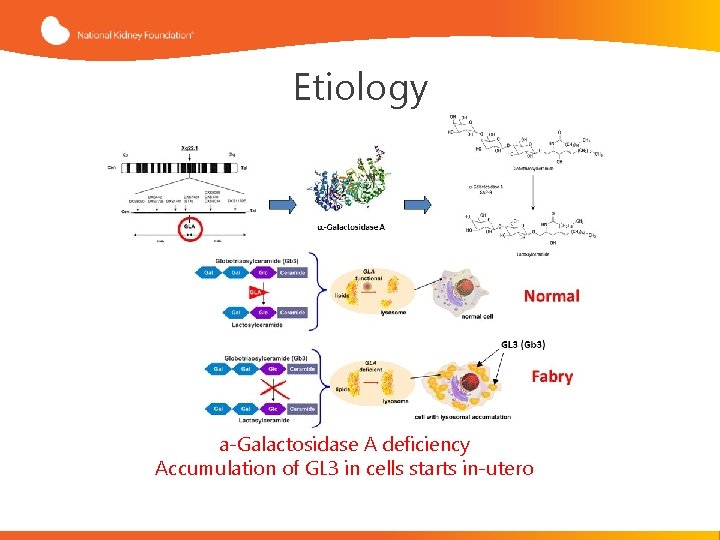
Etiology a-Galactosidase A deficiency Accumulation of GL 3 in cells starts in-utero
Etiology • Currently 790 GLA mutations are recorded (HGMD), ~70% of which are missense/nonsense mutations. The rest are splicing mutations, regulatory mutations, small deletions or insertions, large deletions or insertions, or complex mutations • The majority of these mutations make the enzyme completely or partially non-functional • Non pathological single nucleotide polymorphisms and other sequence variations (VNTR) have been described Human Gene Mutation Database (HGMD). http: //www. hgmd. cf. ac. uk/ac/index. php
Genotype/Phenotype Correlations • High degree of clinical variability both among patients from the same family and among those from unrelated families with the same mutation • Many of the clinical features of Fabry disease are frequently observed in the general population, such as neuropathic and abdominal pain, headache, tinnitus, hearing loss, diarrhea and cardiovascular disease (Fabry disease as a risk factor for commonly encountered pathology)1 • Genetic and environmental modifiers of the phenotype • X-chromosome inactivation impacts the phenotype and natural history of Fabry Disease in females 2 1. Germain D. Fabry disease. Orphanet J Rare Dis. 2010; 5: 30 2. Echevarria L, et al. Clin Genet. 2016: 89: 44 -54.
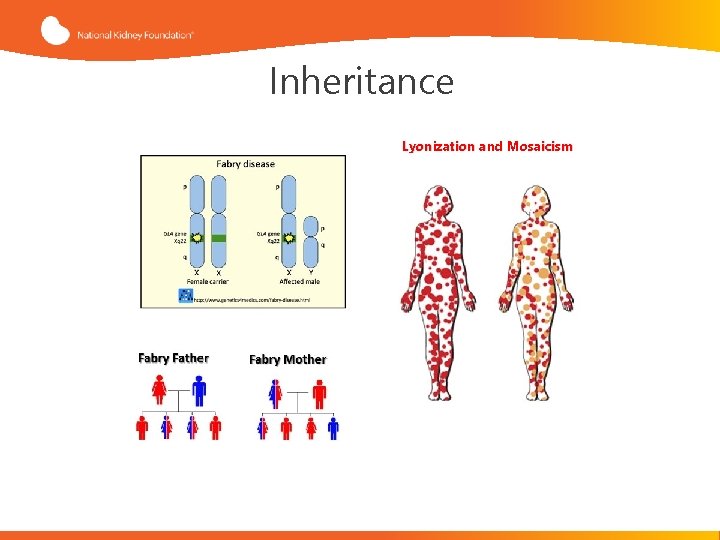
Inheritance Lyonization and Mosaicism

Fabry disease is X-linked, NOT X-linked “recessive” Females with Fabry disease frequently have major organ involvement: Lessons from the Fabry Registry Wilcox et al. Molecular Genetics and Metabolism. 2008; 93: 112 -128.

Complications of Fabry Disease Neurological • • Acroparesthesia o Small nerve fiber degeneration, neuron degeneration in dorsal root ganglia Pain Crisis and Fever o Aversion to exercise Hypohydrosis, anhydrosis, heat intolerance o Small vessel vasculopathy, peripheral neuropathy o Production of tears and saliva reduced in 40% Strokes, Transient Ischemic Attacks o Thrombosis of small arteries o Hypertension secondary to kidney disease Gastrointestinal • • • Abdominal pain Diarrhea Nausea Psychological • • • Anxiety Depression Suicide
Complications of Fabry Disease Cardiac Complications of Fabry Disease • Left Ventricular Hypertrophy/cardiac fibrosis o Usually in patients older than 30 yrs, can lead to CHF and death o In females often fibrosis (MRI) W/O hypertrophy by US • EKG Abnormalities o Short P-R intervals, AV block o Repolarization abnormalities, ST-T changes o Arrhythmias • Aortic root dilatation, valvular disease Cardiac Variant of Fabry Disease • • • Residual AGA activity Presents later in life, no other manifestation Under-recognized: 3% of 230 men with LVH had low AGA activity

Pathology of Fabry Disease Complications Heart Frustaci et al. Circulation 2014 • GL 3 accumulation in cardiac myocytes; left ventricular hypertrophy • GL 3 accumulation in vascular endothelial and smooth muscle cells; ischemic heart disease • Arrhythmias, valvular heart disease

Fabry Cardiomyopathy Echocardiography with LVH and prominent papillary muscles Echocardiographic image of the left-ventricular short-axis of a 50 year old Fabry patient Arrows: Prominent papillary muscle Bar: Hypertrophic Septum (14 mm) Right side: Echocardiographic image of the LV in apical 4 -chamber view LVH, Left ventricular hypertrophy Weidemann F, et al. Curr Pharm Des. 2015; 21: 473 -478.

Pathology of Fabry Disease Complications Nervous System Peripheral Central • GL 3 accumulation in Schwann cells and dorsal root ganglia • The main CNS involvement is due to vasculopathy • Loss of intra-epidermal innervation • GL 3 accumulation in neurons • Predominantly involves small myelinated (Aδ) and unmyelinated (C) fibers. Scott et al. Neurology. 1999; 52: 1249– 54 Schiffmann R et al. Neurological manifestations of Fabry disease. Oxford: Oxford Pharma. Genesis; 2006.
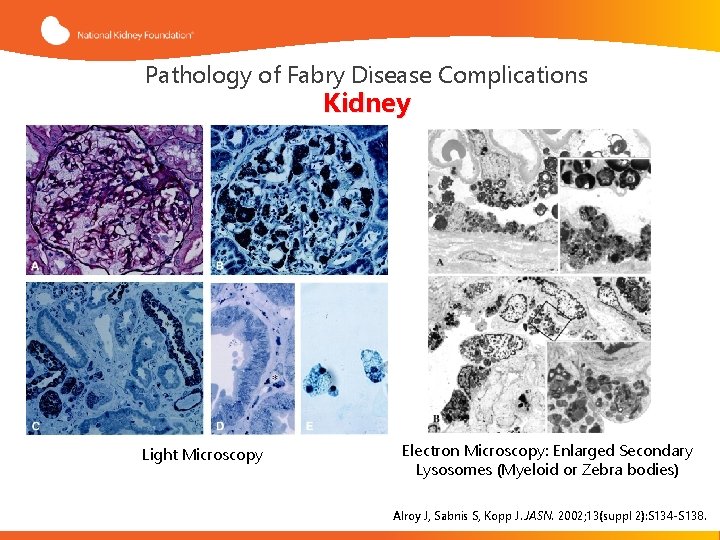
Pathology of Fabry Disease Complications Kidney Light Microscopy Electron Microscopy: Enlarged Secondary Lysosomes (Myeloid or Zebra bodies) Alroy J, Sabnis S, Kopp J. JASN. 2002; 13(suppl 2): S 134 -S 138.

Kidney Injury in Fabry Disease Najafian et al. Kidney International. 2011; 79, 663 -670.

1 9 Evaluation of Fabry Disease

Clinical Manifestations of Fabry Disease • Skin lesions (angiokeratomas) • Pain and burning in the hands and feet (acroparaesthesia) • Fatigue, impaired sweating • Gastrointestinal problems (e. g. , diarrhea, constipation, nausea, and vomiting) • Corneal opacities that progress to a characteristic “whorled” pattern

Angiokeratomas • Dark red/purple lesions, not blanchable • Buttocks, groin, umbilicus, and upper thighs • Adolescence or young adulthood • All ‘classic male’ hemizygotes; 30% of heterozygous females • Biopsy shows dilated capillaries and parakeratosis (dry scaly skin) and endothelial inclusions Kashtan CE. “Alport’s and other familial glomerular syndromes, ” in Comprehensive Clinical Nephrology. Feehally J, Floege J, Johnson RJ, eds, pp. 543 -548, Mosby Elsevier. Philadelphia, PA, USA, 3 rd edition, 2007.
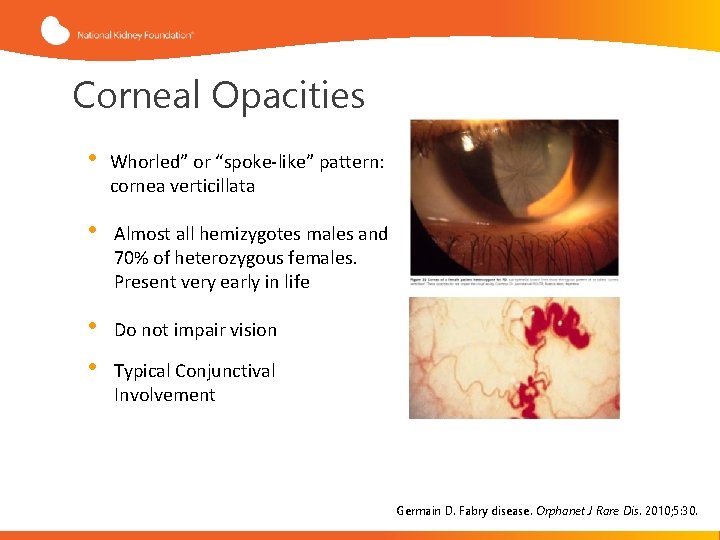
Corneal Opacities • Whorled” or “spoke-like” pattern: cornea verticillata • Almost all hemizygotes males and 70% of heterozygous females. Present very early in life • • Do not impair vision Typical Conjunctival Involvement Germain D. Fabry disease. Orphanet J Rare Dis. 2010; 5: 30.

2 3 Dysmorphic Facies in Fabry disease § § § § Prominent ear lobules Periorbital fullness Bushy eyebrows Recessed forehead Pronounced nasal angle Generous nose Bulbous nasal tip Prominent supraorbital ridges Shallow midface Full lips Prominent nasal bridge Broad alar base Coarse features Posteriorly rotated ears Prognathism Reis M et al. Genet Med 2006: 8: 96 -101.
When to Consider Fabry Disease as a Diagnosis Test ANY patient who has: • A family history of Fabry disease OR • Corneal verticillata (“whorls”) on slit lamp exam Laney DA, Bennett RL, Clarke V, et al. J Genet Counsel. 2013; 22: 555 -564

When to Consider Fabry Disease as a Diagnosis In the absence of these two factors, test patients with at least two of the following features: • Decreased sweating (anhidrosis or hypohidrosis) • Reddish-purple skin rash in the bathing trunk area (angiokeratomas) • Personal and/or family history of kidney failure • Personal or family history of “burning” or “hot” pain in the hands and feet, particularly during fevers (acroparesthesias) • Personal or family history of exercise, heat, or cold intolerance • Patients with sporadic or non-autosomal dominant (no male-tomale) transmission of unexplained cardiac hypertrophy Laney DA, Bennett RL, Clarke V, et al. J Genet Counsel. 2013; 22: 555 -564

Fabry Testing Roadmap Does the patient have clinical features, medical history, laboratory evidence, or family history suggestive of Fabry Disease? Male Patient Female Patient Does the patient have a known family history of Fabry Disease with identified GLA mutation? Order α-galactosidase A enzyme assay of patient ‘s leukocytes The patient has α-gal A activity within normal range The patient is unaffected by Fabry disease The patient has deficient α-gal A activity No Order GLA gene sequencing with reflex testing to GLA duplication/ deletion testing The patient is affected by Fabry disease Yes Targeted sequencing for family mutation Yes No * Was a disease causing mutation identified? *Standard sequencing of GLA will not detect large deletions, large duplications, some intronic mutations, and mutations in the promoter or other regulatory regions. Results must be interpreted in the context of an individual's clinical and/or biochemical profile. Laney DA, Bennett RL, Clarke V, et al. J Genet Counsel. 2013; 22: 555 -564
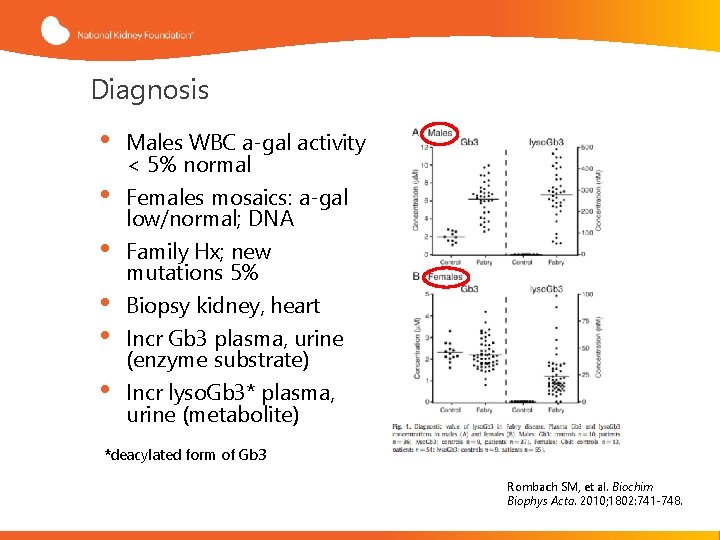
Diagnosis • • • Males WBC a-gal activity < 5% normal Females mosaics: a-gal low/normal; DNA Family Hx; new mutations 5% Biopsy kidney, heart Incr Gb 3 plasma, urine (enzyme substrate) Incr lyso. Gb 3* plasma, urine (metabolite) *deacylated form of Gb 3 Rombach SM, et al. Biochim Biophys Acta. 2010; 1802: 741 -748.

Newborn Screening • Earlier diagnosis promotes timely interventions and the potential to slow the progression of complications such as kidney failure • Newborn screening is detecting a large number of milder mutations, many of unknown clinical significance

Nephrology and Early Identification of Patients • Nephrologists rarely make the diagnosis of Fabry disease other than through surprise findings on kidney biopsy • Undiagnosed Fabry disease patients are typically referred to nephrologists for evaluation of proteinuria and/or decreased GFR • Nephrologists can play a role in early detection, which can present the opportunity to initiate appropriate and timely interventions

Natural History of Fabry Renal Disease • • • Proteinuria is usually the first manifestation. Its earliest appearance is ~14 years of age. Its peak onset is in the 40 s Age of chronic renal insufficiency: 42 (19 -54) Kidney failure develops in most males, usually ~10 years after the onset of proteinuria, and 4± 3 years from onset of CKD 3 Kidney failure can occur as early as 21 years of age, and with the peak incidence in the 50 s More recent registry data found males and females with preserved renal function into their late sixties Branton et al. Medicine 81: 122 -38, 2002 Schiffmann: J Inherit Metab Dis 24 (Suppl 2): 15 -17, 2001 Ortiz et al, NDT 23: 1600 -7, 2008
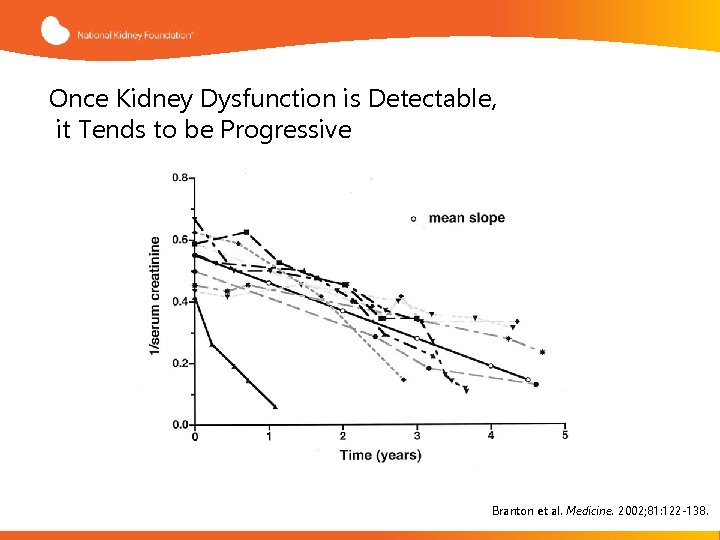
Once Kidney Dysfunction is Detectable, it Tends to be Progressive Branton et al. Medicine. 2002; 81: 122 -138.
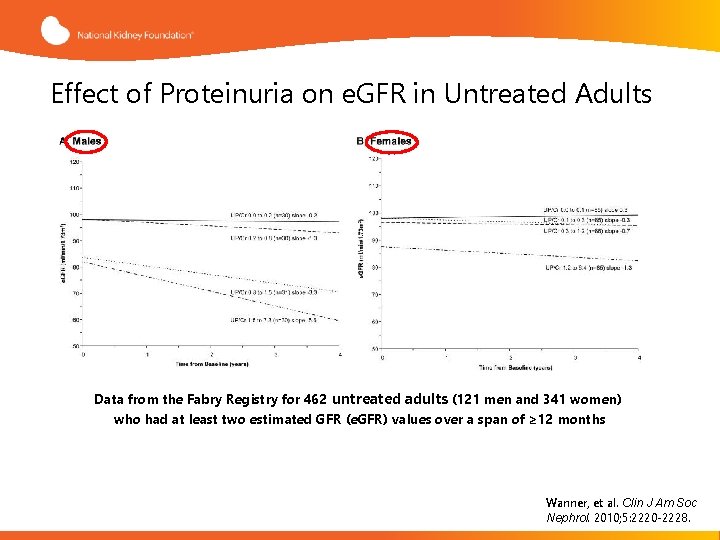
Effect of Proteinuria on e. GFR in Untreated Adults Data from the Fabry Registry for 462 untreated adults (121 men and 341 women) who had at least two estimated GFR (e. GFR) values over a span of ≥ 12 months Wanner, et al. Clin J Am Soc Nephrol. 2010; 5: 2220 -2228.

Assessment of Kidney Function • Regular assessments of kidney function in Fabry patients should include estimates of the glomerular filtration rate (e. GFR), total protein and albumin excretion, and urinary sodium excretion • Urinary protein and urinary albumin excretion, preferably as timed overnight urine collections • If serum creatinine is significantly elevated, e. GFRs may be adequate. If not, e. GFRs are insensitive to early decline and may be better followed by annual or semi-annual measured GFR (e. g, . iohexol clearance) • Biopsy studies have shown that glomerular and vascular changes are present before progression to proteinuria Torra R. Kidney Int Suppl. 2008; 111: S 29 -32. Ortiz A, et al. Nat Clin Pract Nephrol. 2008; 4: 327 -336. Mehta A, et al. QJM. 2010; 103: 641 -659. Tøndel C, et al. Am J Kidney Dis. 2008; 51: 767 -776. Gaspari F, et al. Kidney Int. 2013; 84: 164 -173.

Fabry Disease Management

Management: Multi-disciplinary Approach • Nephrologist • Neurologist • Cardiologist • Geneticist • Pain management • Pediatrician • Psychologist/Psychiatrist • Dermatologist • Ophthalmologist

Enzyme Replacement Therapy (ERT) • Replacement of the deficient/defective enzyme alpha-galactosidase • Two different formulations are available: – Agalsidase alpha (Approved in Europe, not FDA approved) – Agalsidase beta (FDA approved)
Agalsidase Beta • Chinese Hamster Ovary cell line • 1 mg/kg infusion over several hours every two weeks • Premedication • Infusion center followed by home • Life long • Monitor GL 3 and antibodies
Ten-Year Outcome of Enzyme Replacement Therapy With Agalsidase Beta in Patients With Fabry Disease Background: • Analysis of long-term outcomes (median 10 yrs) of patients with classic Fabry disease from the Agalsidase Beta phase 3 clinical trial • First study to classify patients according to their baseline renal involvement (% glomerulosclerosis, UPCR) Methods: • The outcomes (severe clinical events, renal function, cardiac structure) of 52/58 patients with classic Fabry disease from the phase 3 clinical trial and extension study, and the Fabry Registry were evaluated • Disease progression rates for patients with low renal involvement (LRI, n=32) or high renal involvement (HRI, n=20) at baseline were assessed UPCR, urine protein-to creatinine ratio Germain D, et al. J Med Genet. 2015; 52: 353 -358.
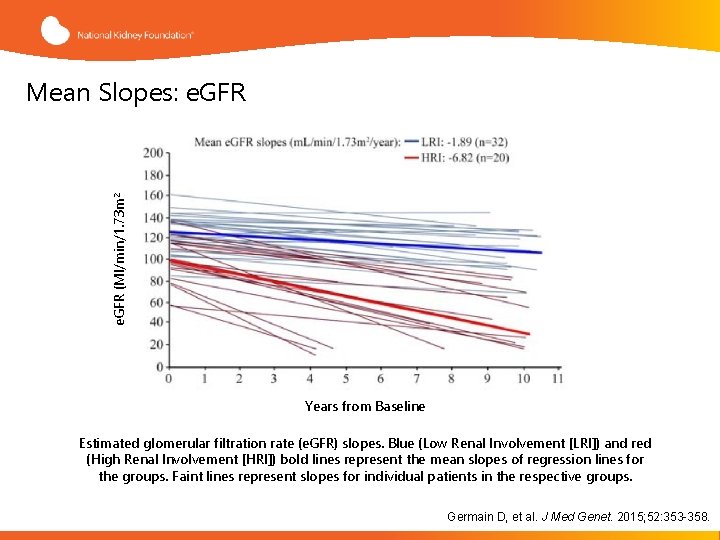
e. GFR (Ml/min/1. 73 m 2 Mean Slopes: e. GFR Years from Baseline Estimated glomerular filtration rate (e. GFR) slopes. Blue (Low Renal Involvement [LRI]) and red (High Renal Involvement [HRI]) bold lines represent the mean slopes of regression lines for the groups. Faint lines represent slopes for individual patients in the respective groups. Germain D, et al. J Med Genet. 2015; 52: 353 -358.
Conclusions and Take Away Points • After 10 -years of Fabrazyme treatment (1 mg/kg/2 wks) in classic Fabry disease: − 94% of the patients were alive − 81% of the patients remained event-free • Long-term treatment decreased the occurrence of severe clinical events • Earlier treatment initiation supports most favorable treatment responses: − Younger patients − Less organ damage Germain D, et al. J Med Genet. 2015; 52: 353 -358.
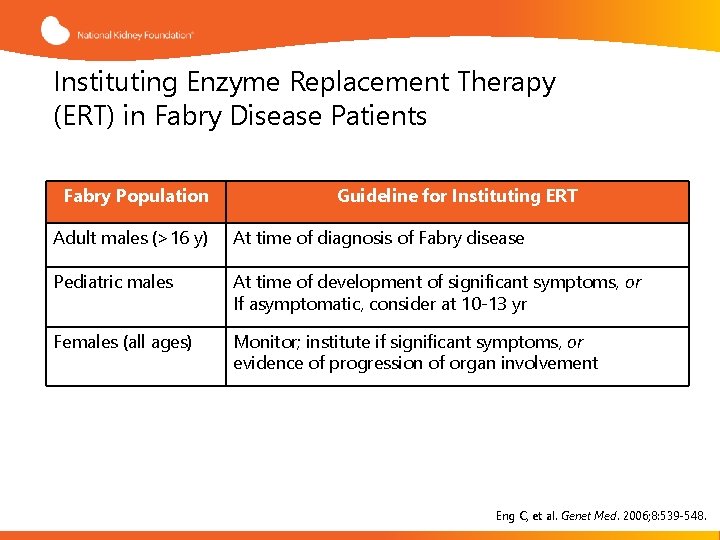
Instituting Enzyme Replacement Therapy (ERT) in Fabry Disease Patients Fabry Population Guideline for Instituting ERT Adult males (>16 y) At time of diagnosis of Fabry disease Pediatric males At time of development of significant symptoms, or If asymptomatic, consider at 10 -13 yr Females (all ages) Monitor; institute if significant symptoms, or evidence of progression of organ involvement Eng C, et al. Genet Med. 2006; 8: 539 -548.
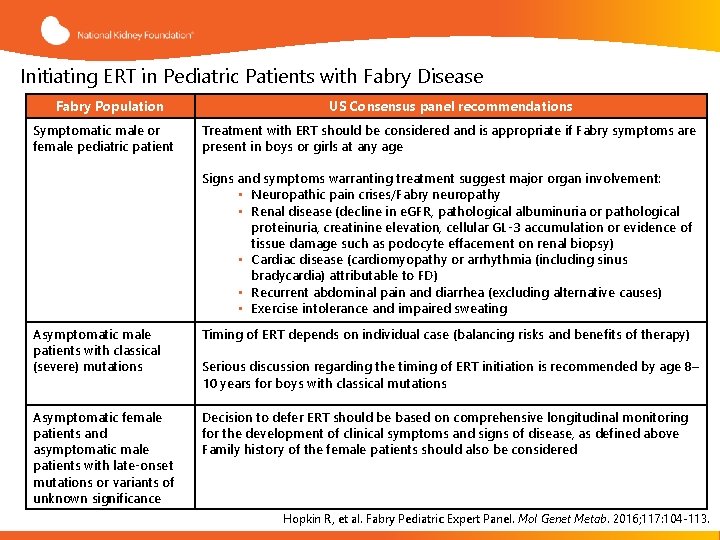
Initiating ERT in Pediatric Patients with Fabry Disease Fabry Population Symptomatic male or female pediatric patient US Consensus panel recommendations Treatment with ERT should be considered and is appropriate if Fabry symptoms are present in boys or girls at any age Signs and symptoms warranting treatment suggest major organ involvement: • Neuropathic pain crises/Fabry neuropathy • Renal disease (decline in e. GFR, pathological albuminuria or pathological proteinuria, creatinine elevation, cellular GL-3 accumulation or evidence of tissue damage such as podocyte effacement on renal biopsy) • Cardiac disease (cardiomyopathy or arrhythmia (including sinus bradycardia) attributable to FD) • Recurrent abdominal pain and diarrhea (excluding alternative causes) • Exercise intolerance and impaired sweating Asymptomatic male patients with classical (severe) mutations Timing of ERT depends on individual case (balancing risks and benefits of therapy) Asymptomatic female patients and asymptomatic male patients with late-onset mutations or variants of unknown significance Decision to defer ERT should be based on comprehensive longitudinal monitoring for the development of clinical symptoms and signs of disease, as defined above Family history of the female patients should also be considered Serious discussion regarding the timing of ERT initiation is recommended by age 8– 10 years for boys with classical mutations Hopkin R, et al. Fabry Pediatric Expert Panel. Mol Genet Metab. 2016; 117: 104 -113.

CKD Management • CKD MBD • CKD Anemia • Acid Base and Electrolytes • Nutritional Vitamin D • High Blood Pressure
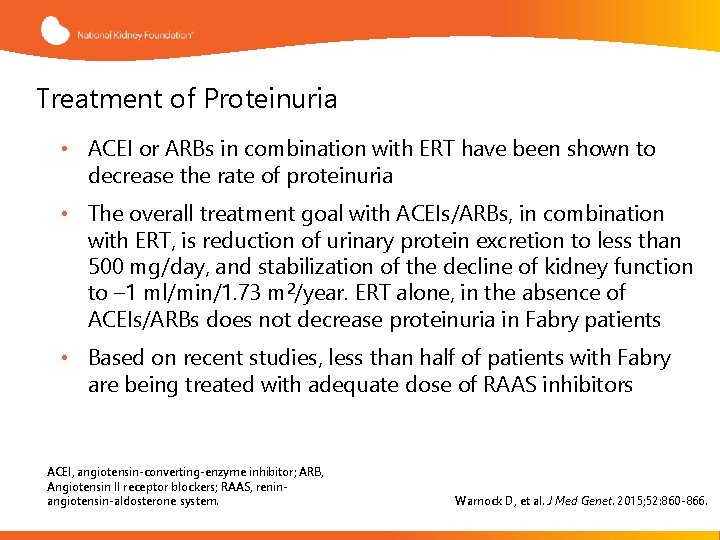
Treatment of Proteinuria • ACEI or ARBs in combination with ERT have been shown to decrease the rate of proteinuria • The overall treatment goal with ACEIs/ARBs, in combination with ERT, is reduction of urinary protein excretion to less than 500 mg/day, and stabilization of the decline of kidney function to – 1 ml/min/1. 73 m 2/year. ERT alone, in the absence of ACEIs/ARBs does not decrease proteinuria in Fabry patients • Based on recent studies, less than half of patients with Fabry are being treated with adequate dose of RAAS inhibitors ACEI, angiotensin-converting-enzyme inhibitor; ARB, Angiotensin II receptor blockers; RAAS, reninangiotensin-aldosterone system. Warnock D, et al. J Med Genet. 2015; 52: 860 -866.

Renal Replacement Therapy • Dialysis – Poorer survival overall – May still benefit from ERT which can be given with dialysis • Transplant – Preferred RRT modality – Living related donors – No benefit in other organs – Recurrence RRT, renal replacement therapy

Pain Management • Pain is one the most important manifestations of Fabry disease • It is mostly because of small fiber dysfunction • Different medications have been recommended for pain management • Generally, studies agree upon starting the medication at low dose, and evaluating tolerability and effectiveness after 2 -3 weeks • Analgesics are also an option, but NSAIDs generally are not considered effective, and can negatively impact kidney function NSAID, nonsteroidal anti-inflammatory drugs Schuller Y, et al. BMC Neurol. 2016; 16: 25
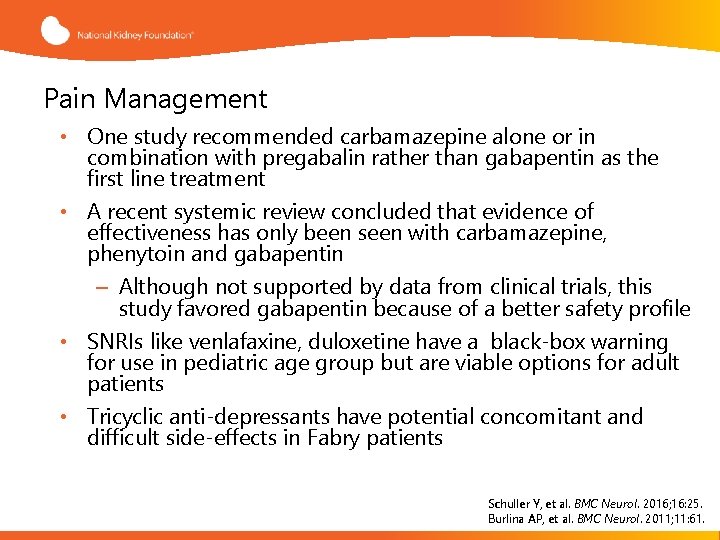
Pain Management • One study recommended carbamazepine alone or in combination with pregabalin rather than gabapentin as the first line treatment • A recent systemic review concluded that evidence of effectiveness has only been seen with carbamazepine, phenytoin and gabapentin – Although not supported by data from clinical trials, this study favored gabapentin because of a better safety profile • SNRIs like venlafaxine, duloxetine have a black-box warning for use in pediatric age group but are viable options for adult patients • Tricyclic anti-depressants have potential concomitant and difficult side-effects in Fabry patients Schuller Y, et al. BMC Neurol. 2016; 16: 25. Burlina AP, et al. BMC Neurol. 2011; 11: 61.
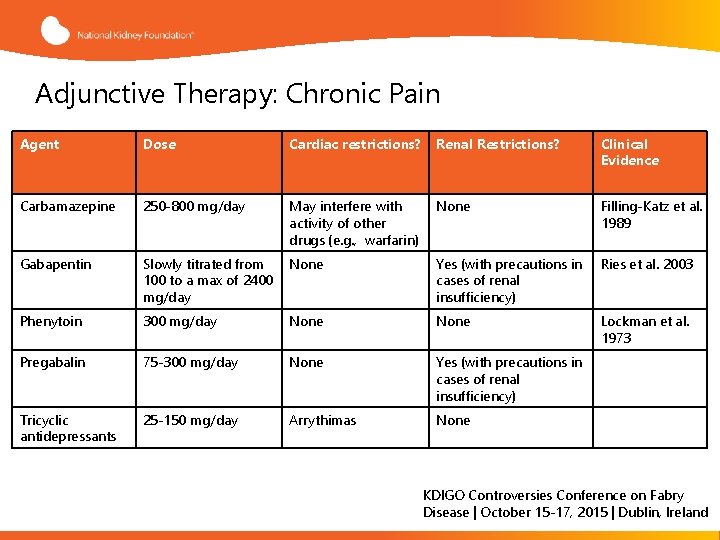
Adjunctive Therapy: Chronic Pain Agent Dose Cardiac restrictions? Renal Restrictions? Clinical Evidence Carbamazepine 250 -800 mg/day May interfere with activity of other drugs (e. g. , warfarin) None Filling-Katz et al. 1989 Gabapentin Slowly titrated from 100 to a max of 2400 mg/day None Yes (with precautions in cases of renal insufficiency) Ries et al. 2003 Phenytoin 300 mg/day None Lockman et al. 1973 Pregabalin 75 -300 mg/day None Yes (with precautions in cases of renal insufficiency) Tricyclic antidepressants 25 -150 mg/day Arrythimas None KDIGO Controversies Conference on Fabry Disease | October 15 -17, 2015 | Dublin, Ireland

Novel Treatments • Several treatments are being looked at for Fabry disease. • Chaperones, Substrate Reduction Therapy (SRT), stem cell transplant and gene therapy among others • Pharmacological chaperones (PC), also known as small molecule ligands, substrate analog competitive inhibitors, or chemical chaperones, can bind and stabilize some mutant forms of a-Gal A in the endoplasmic reticulum.

Summary • Difficult to identify but easy to diagnose • Multisystem progressive disease • Multi-disciplinary approach • Supportive care • Whole family needs to be evaluated and treated
- Slides: 50