Evaluating Probability of Success for Internal Decision Making

Evaluating Probability of Success for Internal Decision Making in Drug Development Narinder Nangia, Ph. D. Martin King, Ph. D. Jane Qian, Ph. D.

Speakers Narinder Nangia, Ph. D. has over 15 years experience as a statistician in the pharmaceutical industry, in both learning and confirming stages of drug development across several therapeutic areas including Pain, Inflammation, CNS and Oncology. For the last few years, he has been advocating the use of Bayesian approaches for decision-making in early drug development. Martin King, Ph. D. has over 10 years experience as a statistician in the pharmaceutical industry, including both early- and late-stage clinical development (Phase 2 -4) in the Antiviral, Oncology, and Dyslipidemia areas. He has presented on the probability of phase 3 success to statistical and non-statistical audiences. Jane Qian, Ph. D. has over 13 years experience as a statistician in the pharmaceutical industry, including both early- and late-stage clinical development (Phase 1 -4) in Cardiovascular and Metabolic, Oncology, Arthritis and Pain, Antiinfective and Antiviral, CNS and Women's health areas. She has been advocating the use of predictive probabilities for early drug development in Oncology. Evaluating probability of success in drug development JSM, 4 August 2008 © 2008 Abbott 2
Course Prerequisite This course is designed for statisticians working in the pharmaceutical or biotechnology industries. Some familiarity with clinical trials and the drug development process is desirable. Participants should have a basic familiarity with hypothesis testing and statistical inference (any first-semester graduate course) and with Bayesian statistics (e. g. , Berry DA. Nat Rev Drug Discov. 2006; 5: 27 -36) Evaluating probability of success in drug development JSM, 4 August 2008 © 2008 Abbott 3
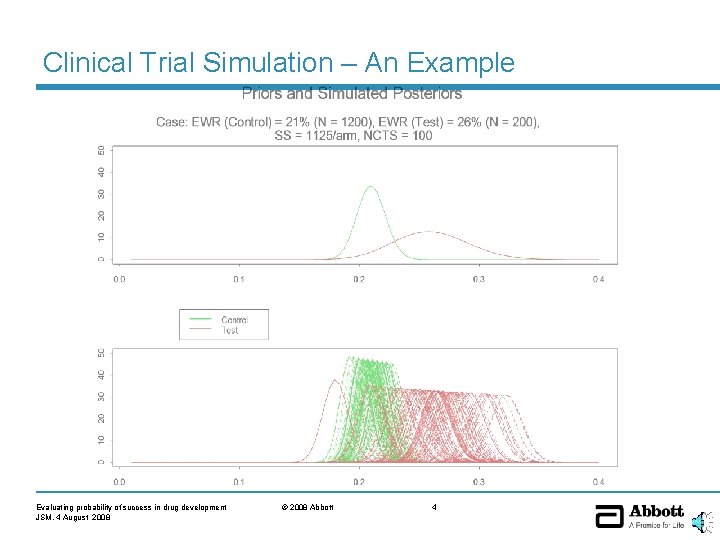
Clinical Trial Simulation – An Example Evaluating probability of success in drug development JSM, 4 August 2008 © 2008 Abbott 4
- Slides: 4