European Patients Academy on Therapeutic Innovation Clinical Trial






- Slides: 13

European Patients’ Academy on Therapeutic Innovation Clinical Trial Designs

Trial design types European Patients’ Academy on Therapeutic Innovation § There are several types of trial designs: Ø Non-randomised controlled trial Ø Randomised controlled trial - Parallel group - Cross-over Ø Single or double blind Ø Superiority or non-inferiority trial 2

Comparisons European Patients’ Academy on Therapeutic Innovation § In a clinical trial design, there a number of different types of comparisons that can be included: Ø Superiority comparison trials demonstrate that the investigational medicine is better than the control. Ø Equivalence comparison trials demonstrate that the endpoint measure is similar (no worse, no better) to the control. Ø Non-inferiority comparison trials demonstrate that the investigational medicine is not worse than the control. Ø Dose-response relationship trials demonstrate various dose parameters including starting dose and maximum dos. 3

Randomisation in clinical trials European Patients’ Academy on Therapeutic Innovation § Randomisation is the process of assigning a trial participant randomly (by chance) to treatment or control groups. § Different tools are used to randomise (closed envelopes, computer sequences, random numbers). § There are two components to randomisation: a) the generation of a random sequence b) Implementation of the random sequence, ideally in a way so that participants are not aware of the sequence. 4

Non-randomised controlled trials European Patients’ Academy on Therapeutic Innovation § Participants are allocated into treatment and control groups by the investigator. § Controls used in non-randomised trials: Ø Concurrent controls: participants matched according to demographics. Ø Historical controls: all participants receive the medicine being studied; the results are either compared to the patient's history (for example a patient living with a chronic illness) or a previous study control group. 5

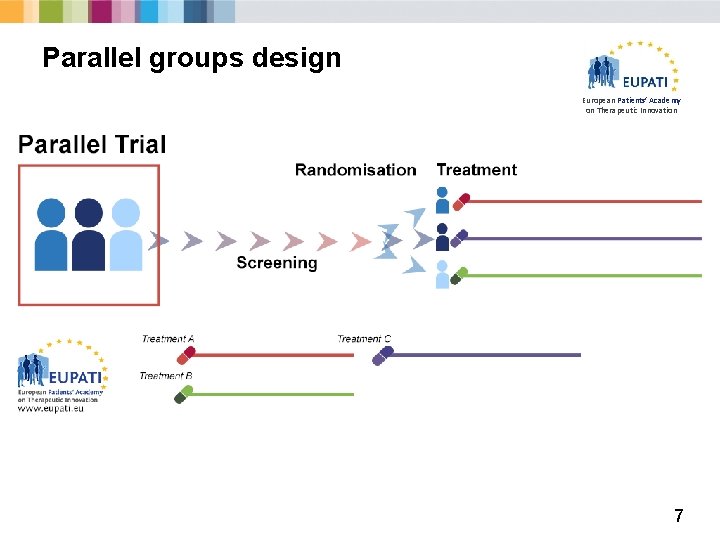
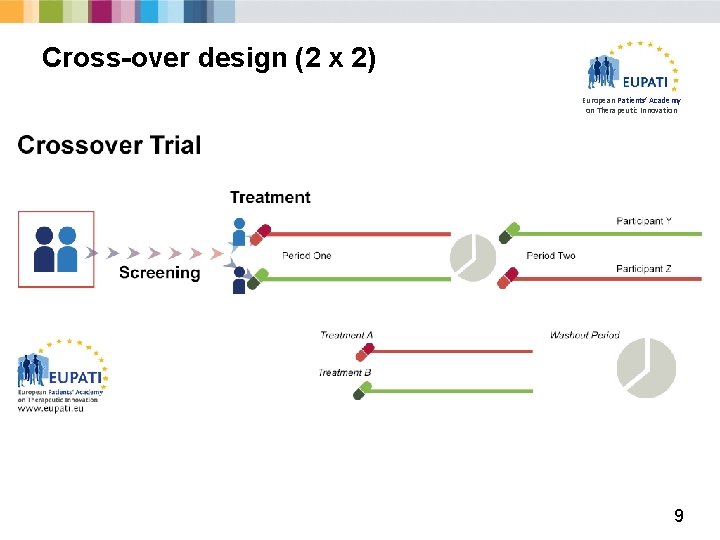
Randomised controlled trials European Patients’ Academy on Therapeutic Innovation § Participants are randomly allocated between treatment and control groups. § Randomisation removes potential for bias. § There are different types of randomised trial designs: 1. Factorial design trials 2. Withdrawal trials 3. Parallel group trials 4. Cross-over trials 6

Parallel groups design European Patients’ Academy on Therapeutic Innovation 7

Evaluation of the parallel groups design Advantages Can be applied to almost any disease European Patients’ Academy on Therapeutic Innovation Challenges Homogenisation of the groups (Especially where different geographical locations are used) Any number of groups can be run simultaneously Groups can be in separate locations 8

Cross-over design (2 x 2) European Patients’ Academy on Therapeutic Innovation 9

Evaluation of the cross-over design European Patients’ Academy on Therapeutic Innovation Advantages Low variance due to treatment and control being the same Challenges Can only be applied to chronic illnesses as treatments are applied one after the other one Can include a number of treatments 10

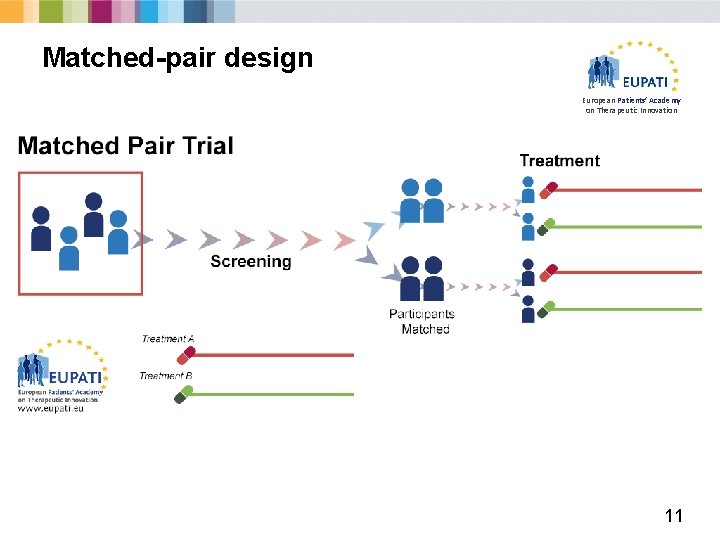
Matched-pair design European Patients’ Academy on Therapeutic Innovation 11

Randomisation using stratification European Patients’ Academy on Therapeutic Innovation § Stratification ensures balanced allocation within each combination. § Studies can be stratified for more than one factor, for example, age and gender. § Common stratification factors include by site, age groups, previous exposure, gender, and lifestyle factors. Smokers Non-smokers Participant No Random arm/group 001 A 003 B 002 B 005 A 004 B 006 B 12

Randomisation using cluster sampling European Patients’ Academy on Therapeutic Innovation § Find suitable geographical areas (e. g. catchment area, city, country, etc. ). § Randomly choose a number of these geographical areas § For each of these chosen geographical areas, choose a proportional subsample from the members of the study population in that area. § Combine these subsamples to get a sample group. 13
 Andreas leischker
Andreas leischker Innovation for the sake of innovation
Innovation for the sake of innovation Incremental innovation vs disruptive innovation
Incremental innovation vs disruptive innovation Clinicaltrials.gov api
Clinicaltrials.gov api Mosaico janssen
Mosaico janssen Companion diagnostic clinical trial
Companion diagnostic clinical trial Morpheus bms
Morpheus bms Ui division of sponsored programs
Ui division of sponsored programs Clinical trial financial management
Clinical trial financial management Prs clinical trial
Prs clinical trial Clinical trial exports
Clinical trial exports Clinical trial budget example
Clinical trial budget example Trofinetide
Trofinetide Clinical trial timeline
Clinical trial timeline

























