EULAR Recommendations for the prevention and management of


















- Slides: 23

EULAR Recommendations for the prevention and management of Antiphospholipid Syndrome in adults

Target population/question • Adult patients with antiphospholipid syndrome (APS) • To develop evidence-based recommendations for the prevention and management of adult APS that will help guide practice and improve quality of care and patient outcomes 2 12/06/2021

Methods/methodological approach • Methods: According to the EULAR Standardized Operating Procedures* Consensual approach Systematic literature research Consensual approach FINAL Recommendations * van der Heijde et al Ann Rheum Dis 2016, 75: 3 -15 3 12/06/2021

Overarching principles • A. Risk stratification in a. PL positive individuals should include determination of the presence of a high-risk a. PL profile (multiple a. PL positivity, lupus anticoagulant, persistently high a. PL titers), prior history of thrombotic and/or obstetric APS, co-existence of other systemic autoimmune diseases such as SLE, and the presence of traditional cardiovascular risk factors. • B. General measures for a. PL positive individuals should include screening for and strict control of cardiovascular risk factors (smoking cessation; management of hypertension, dyslipidemia and diabetes, and regular physical activity) in all individuals and particularly those with a high-risk a. PL profile, screening for and management of venous thrombosis risk factors, and use of low molecular weight heparin in high-risk situations such as surgery, hospitalization, prolonged immobilization and the puerperium. • C. Patient education and counseling on treatment adherence, INR monitoring in patients treated with vitamin K antagonists, use of perioperative bridging therapy with low molecular weight heparin for patients on oral anticoagulants, oral contraceptive use, pregnancy and postpartum period, postmenopausal hormone therapy, and lifestyle recommendations (diet, exercise) are important in the management of APS. 4 12/06/2021

Recommendation 1 • In asymptomatic a. PL carriers (not fulfilling any vascular or obstetric APS classification criteria) with a high-risk a. PL profile with or without traditional risk factors, prophylactic treatment with LDA (75 -100 mg daily) is recommended. Lo. E: 2 a, Go. R: B 5 12/06/2021

Recommendation 2 • In patients with SLE and no history of thrombosis or pregnancy complications: a. with high-risk a. PL profile, prophylactic treatment with LDA is recommended. Lo. E: 2 a, Go. R: B b. with low-risk a. PL profile, prophylactic treatment with LDA may be considered. Lo. E: 2 b, Go. R: C 6 12/06/2021

Recommendation 3 • In non-pregnant women with a history of obstetric APS only (with or without SLE), prophylactic treatment with LDA after adequate risk/benefit evaluation is recommended. Lo. E: 2 a, Go. R: B 7 12/06/2021

Recommendation 4 • In patients with definite APS and first venous thrombosis: a. treatment with VKA with a target INR 2 -3 is recommended. Lo. E: 1 b, Go. R: B b. Rivaroxaban should not be used in patients with triple a. PL positivity due to the high risk of recurrent events. Lo. E: 2 b, Go. R: B DOACs could be considered in patients not able to achieve a target INR despite good adherence to VKA or those with contraindications to VKA (e. g. allergy or intolerance to VKA) Lo. E: 2 b, Go. R: B c. In patients with unprovoked first venous thrombosis, anticoagulation should be continued long-term. Lo. E: 2 b, Go. R: B d. In patients with provoked first venous thrombosis, therapy should be continued for a duration recommended for patients without APS according to international guidelines. Longer anticoagulation could be considered in patients with high-risk a. PL profile in repeated measurements or other risk factors for recurrence. Lo. E: 5, Go. R: D 8 12/06/2021

Recommendation 5 • In patients with definite APS and recurrent venous thrombosis despite treatment with vitamin K antagonists with target INR 2 -3: a. investigation of, and education on, adherence to VKA treatment, along with frequent INR testing should be considered. Lo. E: 5, Go. R: D b. if the target INR 2 -3 had been achieved, addition of LDA, increase of INR target to 3 -4, or change to LMWH may be considered. Lo. E: 4/5, Go. R: D 9 12/06/2021

Recommendation 6 • In patients with definite APS and first arterial thrombosis: a. Treatment with VKA is recommended over treatment with LDA only. Lo. E: 2 b, Go. R: C b. Treatment with VKA with INR 2 -3 or INR 3 -4 is recommended, considering the individual’s risk of bleeding and recurrent thrombosis (Lo. E: 1 b, Go. R: B). Treatment with VKA with INR 2 -3 plus LDA may also be considered (Lo. E: 4, Go. R: C). c. Rivaroxaban should not be used in patients with triple a. PL positivity and arterial events (Lo. E: 1 b, Go. R: B). Based on the current evidence, we do not recommend use of DOACs in patients with definite APS and arterial events, due to high risk of recurrent thrombosis (Lo. E: 5, Go. R: D). 10 12/06/2021

Recommendation 7 • In patients with recurrent arterial thrombosis despite adequate treatment with VKA, after evaluating for other potential causes, an increase of INR target to 3 -4, addition of LDA, or switch to LMWH can be considered. Lo. E: 4/5, Go. R: D 11 12/06/2021

Recommendation 8 • In women with a high-risk a. PL profile but no history of thrombosis or pregnancy complications (with or without SLE), treatment with LDA (75 -100 mg daily) during pregnancy should be considered. Lo. E: 5, Go. R: D 12 12/06/2021

Recommendation 9 • In women with a history of obstetric APS only: a. In women with a history of ≥ 3 recurrent spontaneous miscarriages <10 th week of gestation, and in those with a history of fetal loss (≥ 10 th week of gestation), combination treatment with LDA and heparin at prophylactic dosage during pregnancy is recommended. Lo. E: 2 b, Go. R: B b. In women with a history of delivery <34 weeks of gestation due to eclampsia or severe pre-eclampsia or due to recognised features of placental insufficiency, treatment with LDA or LDA and heparin at prophylactic dosage is recommended considering the individual’s risk profile. Lo. E: 2 b, Go. R: B c. In women with clinical ‘non-criteria’ obstetric APS such as a the presence of two recurrent spontaneous miscarriages <10 th week of gestation, or delivery ≥ 34 weeks of gestation due to severe pre-eclampsia or eclampsia, treatment with LDA alone or in combination with heparin might be considered based on individual’s risk profile. Lo. E: 4, Go. R: D d. In women with obstetric APS treated with prophylactic dose heparin during pregnancy, continuation of heparin at prophylactic dose for 6 weeks after delivery should be considered to reduce the risk of maternal thrombosis. Lo. E: 4, Go. R: C 13 12/06/2021

Recommendation 10 • In women with ‘criteria’ obstetric APS with recurrent pregnancy complications despite combination treatment with LDA and heparin at prophylactic dosage: increase heparin dose to therapeutic dose (Lo. E: 5, Go. R: D), or addition of HCQ (Lo. E: 4, Go. R: D), or low dose prednisolone in the 1 st trimester (Lo. E: 4, Go. R: D), may be considered. Use of intravenous immunoglobulin might be considered in highly selected cases ( Lo. E: 4, Go. R: D). 14 12/06/2021

Recommendation 11 • In women with a history of thrombotic APS, combination treatment of LDA and heparin at therapeutic dosage during pregnancy is recommended. Lo. E: 4, Go. R: D 15 12/06/2021

Recommendation 12 • a. Prompt treatment of infections by early use of anti-infective medications in all a. PL positive individuals, and minimization of interruptions in anticoagulation or low INR level in patients with thrombotic APS, is recommended to help prevent the development of CAPS. Lo. E: 4, Go. R: D b. For first-line treatment of patients with CAPS, combination therapy with glucocorticoids, heparin and plasma exchange or intravenous immunoglobulins is recommended over single agents or other combinations of therapies. Additionally, any triggering factor (e. g. infections, gangrene or malignancy) should be treated accordingly. Lo. E: 5, Go. R: D c. In patients with refractory CAPS, B-cell depletion (e. g. rituximab) or complement inhibition (e. g. eculizumab) therapies may be considered. Lo. E: 4, Go. R: D 16 12/06/2021

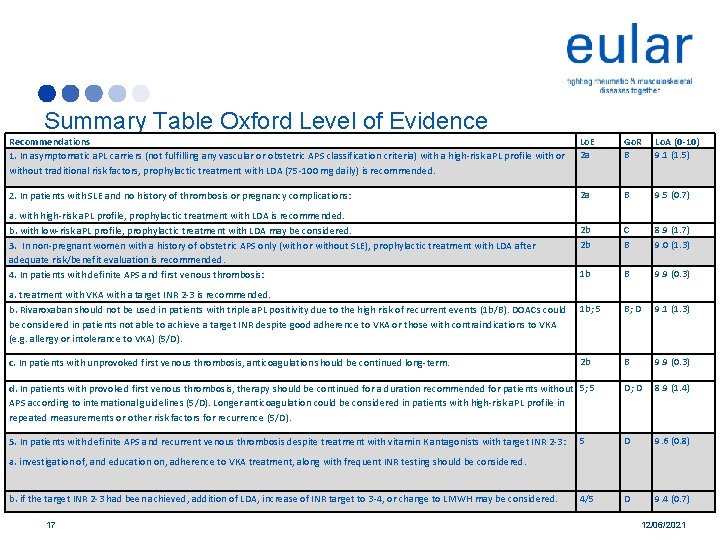
Summary Table Oxford Level of Evidence Recommendations 1. In asymptomatic a. PL carriers (not fulfilling any vascular or obstetric APS classification criteria) with a high-risk a. PL profile with or without traditional risk factors, prophylactic treatment with LDA (75 -100 mg daily) is recommended. Lo. E 2 a Go. R B Lo. A (0 -10) 9. 1 (1. 5) 2. In patients with SLE and no history of thrombosis or pregnancy complications: 2 a B 9. 5 (0. 7) 2 b 2 b C B 8. 9 (1. 7) 9. 0 (1. 3) 1 b B 9. 9 (0. 3) 1 b; 5 B; D 9. 1 (1. 3) 2 b B 9. 9 (0. 3) D; D 8. 9 (1. 4) 5 D 9. 6 (0. 8) 4/5 D 9. 4 (0. 7) a. with high-risk a. PL profile, prophylactic treatment with LDA is recommended. b. with low-risk a. PL profile, prophylactic treatment with LDA may be considered. 3. In non-pregnant women with a history of obstetric APS only (with or without SLE), prophylactic treatment with LDA after adequate risk/benefit evaluation is recommended. 4. In patients with definite APS and first venous thrombosis: a. treatment with VKA with a target INR 2 -3 is recommended. b. Rivaroxaban should not be used in patients with triple a. PL positivity due to the high risk of recurrent events (1 b/B). DOACs could be considered in patients not able to achieve a target INR despite good adherence to VKA or those with contraindications to VKA (e. g. allergy or intolerance to VKA) (5/D). c. In patients with unprovoked first venous thrombosis, anticoagulation should be continued long-term. d. In patients with provoked first venous thrombosis, therapy should be continued for a duration recommended for patients without 5; 5 APS according to international guidelines (5/D). Longer anticoagulation could be considered in patients with high-risk a. PL profile in repeated measurements or other risk factors for recurrence (5/D). 5. In patients with definite APS and recurrent venous thrombosis despite treatment with vitamin K antagonists with target INR 2 -3: a. investigation of, and education on, adherence to VKA treatment, along with frequent INR testing should be considered. b. if the target INR 2 -3 had been achieved, addition of LDA, increase of INR target to 3 -4, or change to LMWH may be considered. 17 12/06/2021

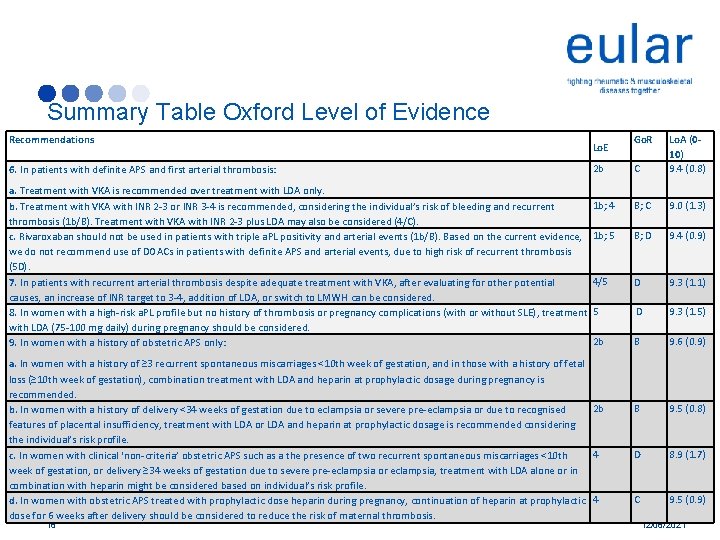
Summary Table Oxford Level of Evidence Recommendations 6. In patients with definite APS and first arterial thrombosis: a. Treatment with VKA is recommended over treatment with LDA only. b. Treatment with VKA with INR 2 -3 or INR 3 -4 is recommended, considering the individual’s risk of bleeding and recurrent thrombosis (1 b/B). Treatment with VKA with INR 2 -3 plus LDA may also be considered (4/C). c. Rivaroxaban should not be used in patients with triple a. PL positivity and arterial events (1 b/B). Based on the current evidence, we do not recommend use of DOACs in patients with definite APS and arterial events, due to high risk of recurrent thrombosis (5 D). 7. In patients with recurrent arterial thrombosis despite adequate treatment with VKA, after evaluating for other potential causes, an increase of INR target to 3 -4, addition of LDA, or switch to LMWH can be considered. 8. In women with a high-risk a. PL profile but no history of thrombosis or pregnancy complications (with or without SLE), treatment with LDA (75 -100 mg daily) during pregnancy should be considered. 9. In women with a history of obstetric APS only: 2 b C Lo. A (010) 9. 4 (0. 8) 1 b; 4 B; C 9. 0 (1. 3) 1 b; 5 B; D 9. 4 (0. 9) 4/5 D 9. 3 (1. 1) 5 D 9. 3 (1. 5) 2 b B 9. 6 (0. 9) B 9. 5 (0. 8) D 8. 9 (1. 7) C 9. 5 (0. 9) Lo. E a. In women with a history of ≥ 3 recurrent spontaneous miscarriages <10 th week of gestation, and in those with a history of fetal loss (≥ 10 th week of gestation), combination treatment with LDA and heparin at prophylactic dosage during pregnancy is recommended. 2 b b. In women with a history of delivery <34 weeks of gestation due to eclampsia or severe pre-eclampsia or due to recognised features of placental insufficiency, treatment with LDA or LDA and heparin at prophylactic dosage is recommended considering the individual’s risk profile. 4 c. In women with clinical ‘non-criteria’ obstetric APS such as a the presence of two recurrent spontaneous miscarriages <10 th week of gestation, or delivery ≥ 34 weeks of gestation due to severe pre-eclampsia or eclampsia, treatment with LDA alone or in combination with heparin might be considered based on individual’s risk profile. d. In women with obstetric APS treated with prophylactic dose heparin during pregnancy, continuation of heparin at prophylactic 4 dose for 6 weeks after delivery should be considered to reduce the risk of maternal thrombosis. 18 Go. R 12/06/2021

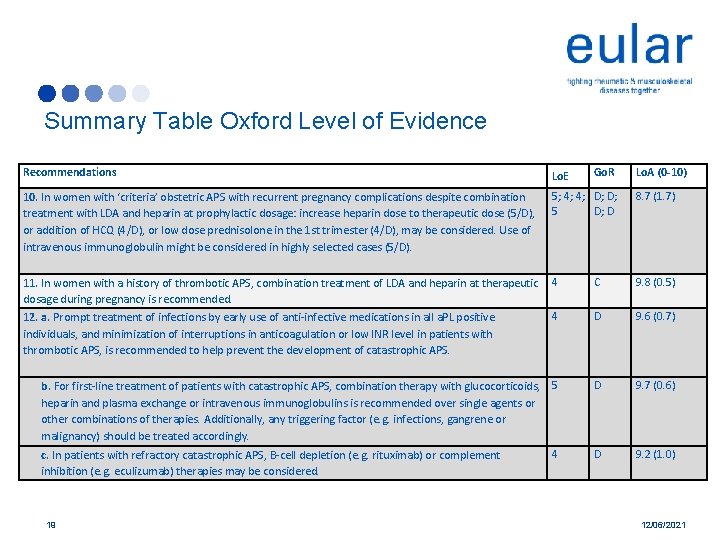
Summary Table Oxford Level of Evidence Recommendations Lo. E 10. In women with ‘criteria’ obstetric APS with recurrent pregnancy complications despite combination treatment with LDA and heparin at prophylactic dosage: increase heparin dose to therapeutic dose (5/D), or addition of HCQ (4/D), or low dose prednisolone in the 1 st trimester (4/D), may be considered. Use of intravenous immunoglobulin might be considered in highly selected cases (5/D). 5; 4; 4; D; D; 5 D; D 8. 7 (1. 7) 11. In women with a history of thrombotic APS, combination treatment of LDA and heparin at therapeutic dosage during pregnancy is recommended. 12. a. Prompt treatment of infections by early use of anti-infective medications in all a. PL positive individuals, and minimization of interruptions in anticoagulation or low INR level in patients with thrombotic APS, is recommended to help prevent the development of catastrophic APS. 4 C 9. 8 (0. 5) 4 D 9. 6 (0. 7) b. For first-line treatment of patients with catastrophic APS, combination therapy with glucocorticoids, 5 heparin and plasma exchange or intravenous immunoglobulins is recommended over single agents or other combinations of therapies. Additionally, any triggering factor (e. g. infections, gangrene or malignancy) should be treated accordingly. D 9. 7 (0. 6) 4 D 9. 2 (1. 0) c. In patients with refractory catastrophic APS, B-cell depletion (e. g. rituximab) or complement inhibition (e. g. eculizumab) therapies may be considered. 19 Go. R Lo. A (0 -10) 12/06/2021

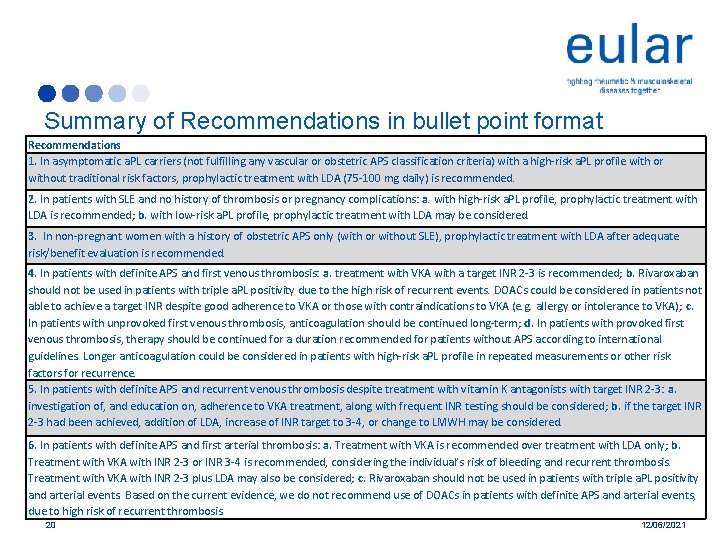
Summary of Recommendations in bullet point format Recommendations 1. In asymptomatic a. PL carriers (not fulfilling any vascular or obstetric APS classification criteria) with a high-risk a. PL profile with or without traditional risk factors, prophylactic treatment with LDA (75 -100 mg daily) is recommended. 2. In patients with SLE and no history of thrombosis or pregnancy complications: a. with high-risk a. PL profile, prophylactic treatment with LDA is recommended; b. with low-risk a. PL profile, prophylactic treatment with LDA may be considered. 3. In non-pregnant women with a history of obstetric APS only (with or without SLE), prophylactic treatment with LDA after adequate risk/benefit evaluation is recommended. 4. In patients with definite APS and first venous thrombosis: a. treatment with VKA with a target INR 2 -3 is recommended; b. Rivaroxaban should not be used in patients with triple a. PL positivity due to the high risk of recurrent events. DOACs could be considered in patients not able to achieve a target INR despite good adherence to VKA or those with contraindications to VKA (e. g. allergy or intolerance to VKA); c. In patients with unprovoked first venous thrombosis, anticoagulation should be continued long-term; d. In patients with provoked first venous thrombosis, therapy should be continued for a duration recommended for patients without APS according to international guidelines. Longer anticoagulation could be considered in patients with high-risk a. PL profile in repeated measurements or other risk factors for recurrence. 5. In patients with definite APS and recurrent venous thrombosis despite treatment with vitamin K antagonists with target INR 2 -3: a. investigation of, and education on, adherence to VKA treatment, along with frequent INR testing should be considered; b. if the target INR 2 -3 had been achieved, addition of LDA, increase of INR target to 3 -4, or change to LMWH may be considered. 6. In patients with definite APS and first arterial thrombosis: a. Treatment with VKA is recommended over treatment with LDA only; b. Treatment with VKA with INR 2 -3 or INR 3 -4 is recommended, considering the individual’s risk of bleeding and recurrent thrombosis. Treatment with VKA with INR 2 -3 plus LDA may also be considered; c. Rivaroxaban should not be used in patients with triple a. PL positivity and arterial events. Based on the current evidence, we do not recommend use of DOACs in patients with definite APS and arterial events, due to high risk of recurrent thrombosis. 20 12/06/2021

Summary of Recommendations in bullet point format Recommendations 7. In patients with recurrent arterial thrombosis despite adequate treatment with VKA, after evaluating for other potential causes, an increase of INR target to 3 -4, addition of LDA, or switch to LMWH can be considered. 8. In women with a high-risk a. PL profile but no history of thrombosis or pregnancy complications (with or without SLE), treatment with LDA (75100 mg daily) during pregnancy should be considered. 9. In women with a history of obstetric APS only: a. In women with a history of ≥ 3 recurrent spontaneous miscarriages <10 th week of gestation, and in those with a history of fetal loss (≥ 10 th week of gestation), combination treatment with LDA and heparin at prophylactic dosage during pregnancy is recommended; b. In women with a history of delivery <34 weeks of gestation due to eclampsia or severe pre-eclampsia or due to recognised features of placental insufficiency, treatment with LDA or LDA and heparin at prophylactic dosage is recommended considering the individual’s risk profile; c. In women with clinical ‘non-criteria’ obstetric APS such as a the presence of two recurrent spontaneous miscarriages <10 th week of gestation, or delivery ≥ 34 weeks of gestation due to severe pre-eclampsia or eclampsia, treatment with LDA alone or in combination with heparin might be considered based on individual’s risk profile; d. In women with obstetric APS treated with prophylactic dose heparin during pregnancy, continuation of heparin at prophylactic dose for 6 weeks after delivery should be considered to reduce the risk of maternal thrombosis. 10. In women with ‘criteria’ obstetric APS with recurrent pregnancy complications despite combination treatment with LDA and heparin at prophylactic dosage: increase heparin dose to therapeutic dose, or addition of HCQ, or low dose prednisolone in the 1 st trimester, may be considered. Use of intravenous immunoglobulin might be considered in highly selected cases. 11. In women with a history of thrombotic APS, combination treatment of LDA and heparin at therapeutic dosage during pregnancy is recommended. 12. a. Prompt treatment of infections by early use of anti-infective medications in all a. PL positive individuals, and minimization of interruptions in anticoagulation or low INR level in patients with thrombotic APS, is recommended to help prevent the development of catastrophic APS; b. For first-line treatment of patients with catastrophic APS, combination therapy with glucocorticoids, heparin and plasma exchange or intravenous immunoglobulins is recommended over single agents or other combinations of therapies. Additionally, any triggering factor (e. g. infections, gangrene or malignancy) should be treated accordingly; c. In patients with refractory catastrophic APS, B-cell depletion (e. g. 21 12/06/2021 rituximab) or complement inhibition (e. g. eculizumab) therapies may be considered.

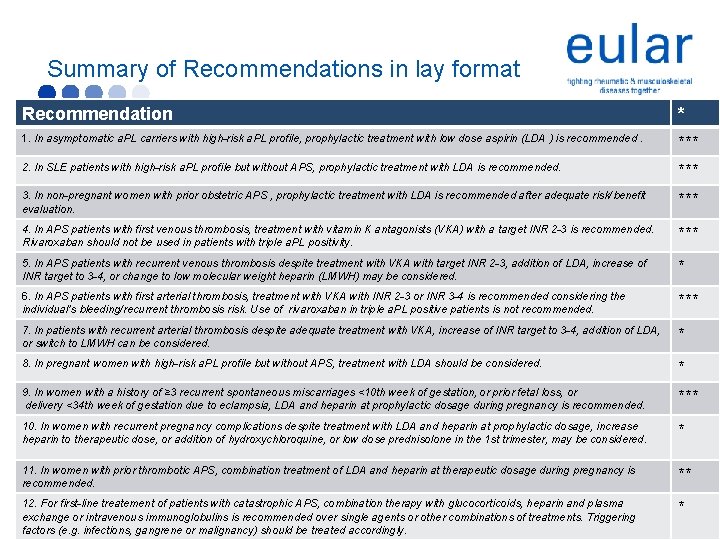
Summary of Recommendations in lay format Recommendation * 1. In asymptomatic a. PL carriers with high-risk a. PL profile, prophylactic treatment with low dose aspirin (LDA ) is recommended. *** 2. In SLE patients with high-risk a. PL profile but without APS, prophylactic treatment with LDA is recommended. *** 3. In non-pregnant women with prior obstetric APS , prophylactic treatment with LDA is recommended after adequate risk/benefit evaluation. *** 4. In APS patients with first venous thrombosis, treatment with vitamin K antagonists (VKA) with a target INR 2 -3 is recommended. Rivaroxaban should not be used in patients with triple a. PL positivity. *** 5. In APS patients with recurrent venous thrombosis despite treatment with VKA with target INR 2 -3, addition of LDA, increase of INR target to 3 -4, or change to low molecular weight heparin (LMWH) may be considered. * 6. In APS patients with first arterial thrombosis, treatment with VKA with INR 2 -3 or INR 3 -4 is recommended considering the individual’s bleeding/recurrent thrombosis risk. Use of rivaroxaban in triple a. PL positive patients is not recommended. *** 7. In patients with recurrent arterial thrombosis despite adequate treatment with VKA, increase of INR target to 3 -4, addition of LDA, or switch to LMWH can be considered. * 8. In pregnant women with high-risk a. PL profile but without APS, treatment with LDA should be considered. * 9. In women with a history of ≥ 3 recurrent spontaneous miscarriages <10 th week of gestation, or prior fetal loss, or delivery <34 th week of gestation due to eclampsia, LDA and heparin at prophylactic dosage during pregnancy is recommended. *** 10. In women with recurrent pregnancy complications despite treatment with LDA and heparin at prophylactic dosage, increase heparin to therapeutic dose, or addition of hydroxychloroquine, or low dose prednisolone in the 1 st trimester, may be considered. * 11. In women with prior thrombotic APS, combination treatment of LDA and heparin at therapeutic dosage during pregnancy is recommended. ** 12. For first-line treatement of patients with catastrophic APS, combination therapy with glucocorticoids, heparin and plasma exchange or intravenous immunoglobulins is recommended over single agents or other combinations of treatments. Triggering factors (e. g. infections, gangrene or malignancy) should be treated accordingly. *

Acknowledgements • The task force members would like to thank the European League Against Rheumatism for financial support. The panel is grateful for the administrative and logistical support from EULAR Executive secretariat and especially from Patrizia Jud, Εxecutive Αssistant. • Convenor: Maria G. Tektonidou • Co-Convenor: Angela Tincani • Methodologist: Michael M. Ward • Fellows for literature search: Laura Andreoli, Maarten Limper • Task Force Members : Z. Amoura, R. Cervera, N. Costedoat-Chalumeau, M. J. Cuadrado, T. Dörner, R. Ferrer, K. Hambly, M. Khamashta, J. King, F. Marchiori, P. L. Meroni, M. Mosca, V. Pengo, L. Raio, G. Ruiz-Irastorza, Y. Shoenfeld, L. Stojanovich, E. Svenungsson, D. Wahl 23 12/06/2021