ETHYLENE OXIDE STERILIZATION VALIDATION Pacific Bio Labs Inc

ETHYLENE OXIDE STERILIZATION VALIDATION Pacific Bio. Labs Inc. (510) 964 -9000 info@Pacific. Bio. Labs. com

EO ADVANTAGES n Highly effective against most microbes n Highly diffusive n Compatible with a wide variety of materials in devices and packaging 2

EO DISADVANTAGES n Complex process n Longer turn-around times · BI Testing · Residual disipation n Safety concerns · Flammable · Explosive n OSHA concerns · Carcinogen n EPA concerns · Emissions 3

DETERMINE THE STANDARD n AAMI/ISO 11135 -01 4 ed “Sterilization of health care products – Ethylene oxide - Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices” n Europe – EN 550 4

EO GUIDANCE DOCUMENTS n AAMI Technical Information Reports (TIR’s) · 14 Contract sterilization · 15 Equipment · 16 Microbiological aspects · 20 Parametric release · 28 Product adoption and process equivalency 5
EO PROCESSING STEPS n Preconditioning/conditioning · Exposure to RH and temperature · Ensure uniformity of these conditions n Sterilization cycle · Exposure to EO gas n Aeration · Dissipation of remaining gases 6
DECISIVE PROCESS PARAMETERS n Gas concentration >400 mg/L n Temperature ~100 – 140ºC n Relative humidity ~35 – 80% n Exposure (dwell) time 2 – 10 hours 7
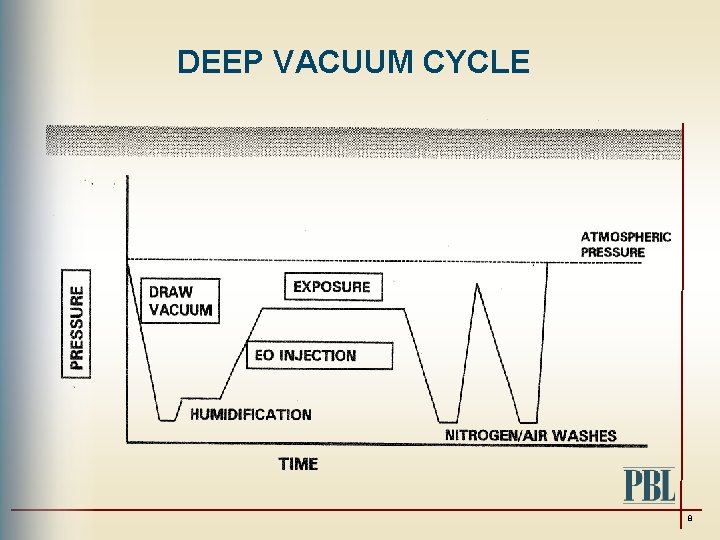
DEEP VACUUM CYCLE 8
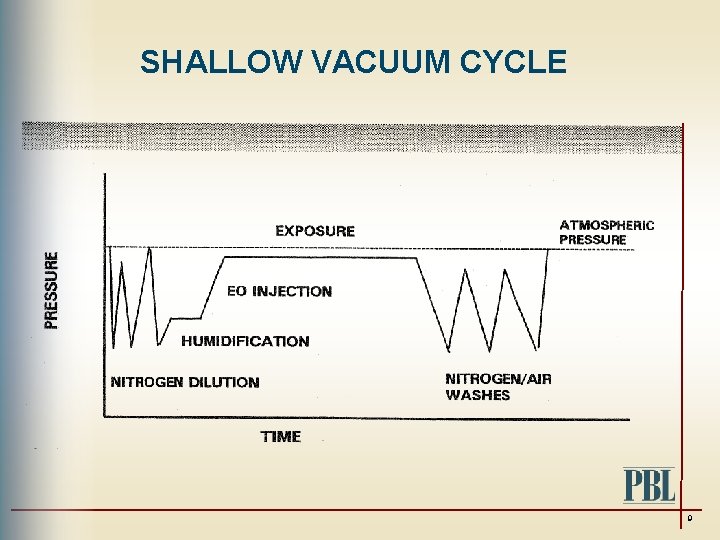
SHALLOW VACUUM CYCLE 9
FACTORS AFFECTING CYCLE SUCCESS n Bioburden n Product/package properties n Loading configuration n Cycle parameters 10

EO VALIDATION OVERVIEW n Process development n Product compatibility n Commissioning n PQ – Physical n PQ – Microbiological n Certification n Revalidation 11
PROCESS CONTROL n Must assure that validated process parameters are met · Temperature · RH · Gas concentration n Biological indicators are used to demonstrate lethality n Microprocessors are used to control process 12
RELEASE MECHANISMS n Documentation showing that processing specification are met n Successful results of tests · Sterility of BI · EO residues · Packaging · Pyrogens 13
PARAMETRIC RELEASE n BIs not used in release n Validation more involved n Routine control more rigorous n AAMI TIR 20: 2001 “Parametric release for ethylene oxide sterilization” 14
PRODUCT COMPATIBILITY n Post sterilization testing for · Device functionality · Package integrity and strength · Residue dissipation rates · Impact of re-sterilization 15
COMMISSIONING n Equipment specifications/diagram n Calibration records n Profiles for · Preconditioning (temp. and RH) · Aeration rooms (temp. ) · Empty chamber temperature distribution 16
PQ - PHYSICAL n Profiles within loaded preconditioning and aeration areas n Loaded chamber temperature distribution studies n Diagrams showing load configuration, thermocouple and BI placement 17
PQ - MICROBIOLOGICAL n Records of performance runs (sub-lethal, half, and full cycles) n Diagrams of load configuration with BI and thermocouple placement n BI test result n Sterility test result of product n B/F testing 18
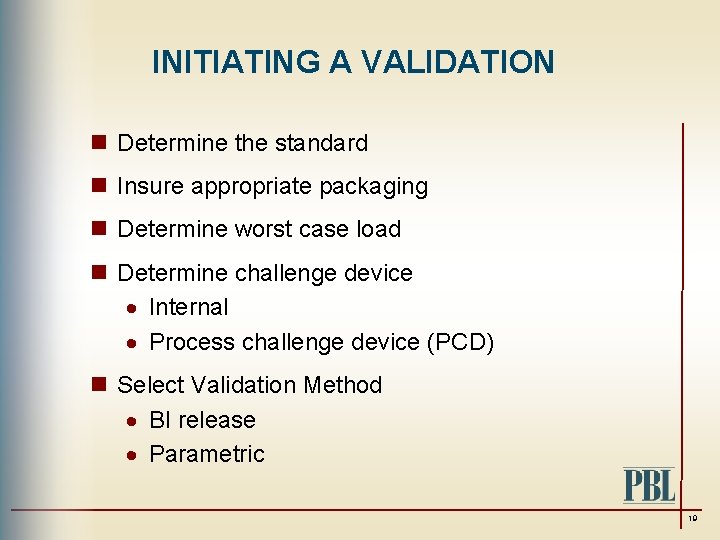
INITIATING A VALIDATION n Determine the standard n Insure appropriate packaging n Determine worst case load n Determine challenge device · Internal · Process challenge device (PCD) n Select Validation Method · BI release · Parametric 19

CHALLENGE DEVICES n Internal Challenge Device (ICD) · Most difficult to sterilize devices seeded with a BI in the most difficult to sterilize location n PCD · An external BI test pack that replaces the internal challenge device · Should be an equal or more difficult challenge to the process than the ICD · Developed using comparative resistance studies 20
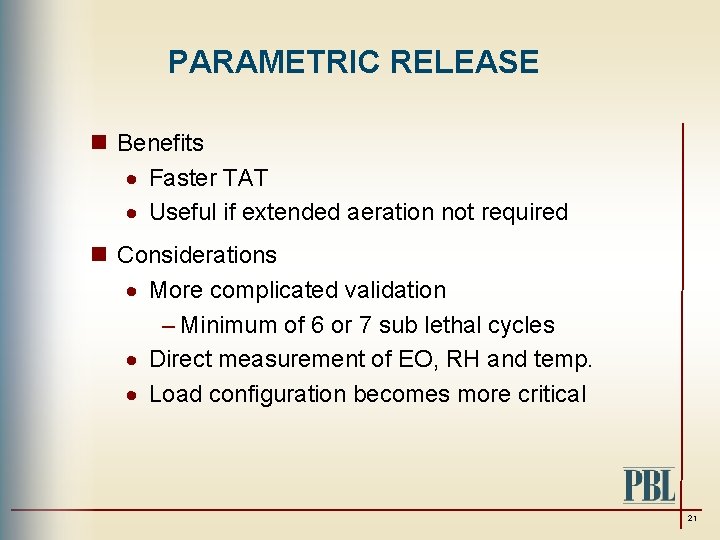
PARAMETRIC RELEASE n Benefits · Faster TAT · Useful if extended aeration not required n Considerations · More complicated validation – Minimum of 6 or 7 sub lethal cycles · Direct measurement of EO, RH and temp. · Load configuration becomes more critical 21
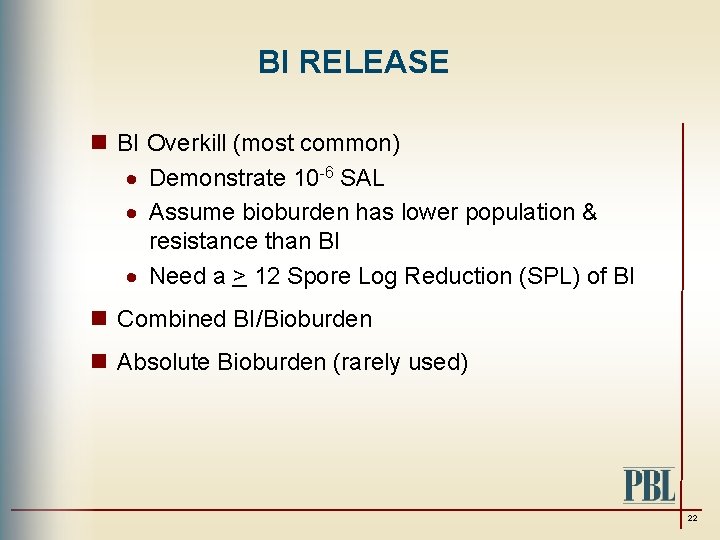
BI RELEASE n BI Overkill (most common) · Demonstrate 10 -6 SAL · Assume bioburden has lower population & resistance than BI · Need a > 12 Spore Log Reduction (SPL) of BI n Combined BI/Bioburden n Absolute Bioburden (rarely used) 22
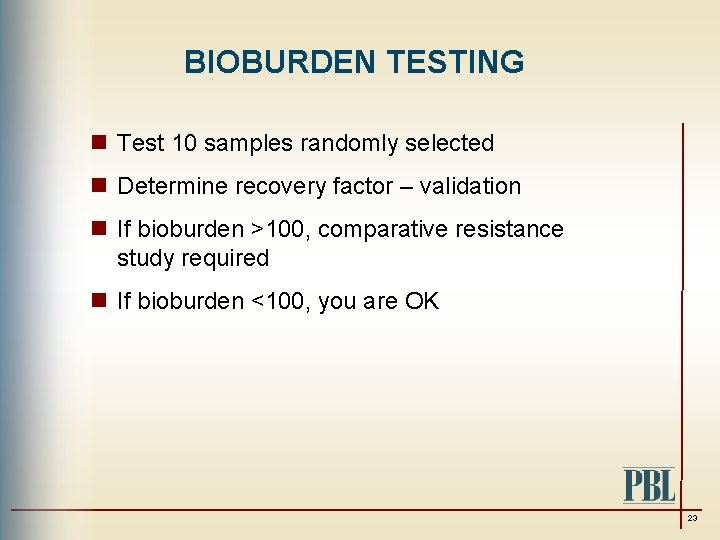
BIOBURDEN TESTING n Test 10 samples randomly selected n Determine recovery factor – validation n If bioburden >100, comparative resistance study required n If bioburden <100, you are OK 23
SAMPLE PLACEMENT n Protocol must detail the number and location of all samples in load · BI’s · Product sterility (if applicable) · ETO residuals · Product functionality · Package integrity · LAL 24
VALIDATION CYCLES n Fractional cycles n Half cycles n Full cycles 25
FRACTIONAL CYCLE n Must be run when bioburden >100 and no comparative resistance studies are performed n Desired cycle time must results in some positive BI and sterile product in sterility tests n A minimum of 20 product sterility samples (10 TSB, 10 FTM) n Product sterility samples must be placed adjacent to BI 26

HALF CYCLES n Three half cycles must be run in production chamber with a gas dwell time half the full cycle dwell time n The following must be placed in load · Temperature and humidity sensors · Internal BI · External BI (optional) · Product sterility samples if comparative resistance studies not done or inconclusive 27
FULL CYCLE n A minimum of one full cycle is required for the Micro PQ n Three cycles are required to meet residual requirements n The following samples are included · EO residual · Product functionality · Packaging integrity · External BI (routine release BI) · LAL 28

EO RESIDUAL TESTING n 1 - 3 samples of each type should be tested at a minimum of 3 time intervals from processing (Ex. 1, 3, & 5 days) n This must be done after 3 full cycles n Testing for EO and ECH n Samples must be shipped frozen 29
ACCEPTANCE CRITERIA n Bioburden must be in control n Product sterility all neg after half cycles n Acceptable B&F test n BI Testing · Fractional cycle - some should grow · Half cycle - all negative · Full cycle - all negative 30
ACCEPTANCE CRITERIA (cont. ) n Temperature sensors <10°C n Humidity sensors <30% n EO residual n Product functionality n Package integrity n LAL 31

REVALIDATION n Annually the status of the sterilization validation must be reviewed n Physical and biological revalidation must be conducted every two years n Inspection of · Product design and packaging · Chamber performance, calibration and maintenance 32

REVALIDATION (cont. ) n If there have been changes in product design, packaging, or chamber performance, a physical and biological revalidation may be required n Validation should consist of a minimum of one half cycle and one full cycle 33
REFERENCES n AAMI/ISO 11135 -01 4 ed. Sterilization of health care products- Ethylene oxide- Part 1: requirements for the development, validation and routine control of a sterilization process from medical devices n AAMI TIR No. 16: 2000, Process development and performance qualification for ethylene oxide sterilization – Microbiological aspects n AAMI TIR No. 29: 2001, Parametric release for ethylene oxide sterilization n AAMI TIR 28: 2001, Product adoption and process equivalency for ethylene oxide sterilization 34

THANK YOU Q&A
- Slides: 35