Ethers ROR or ROR Nomenclature simple ethers are





- Slides: 17

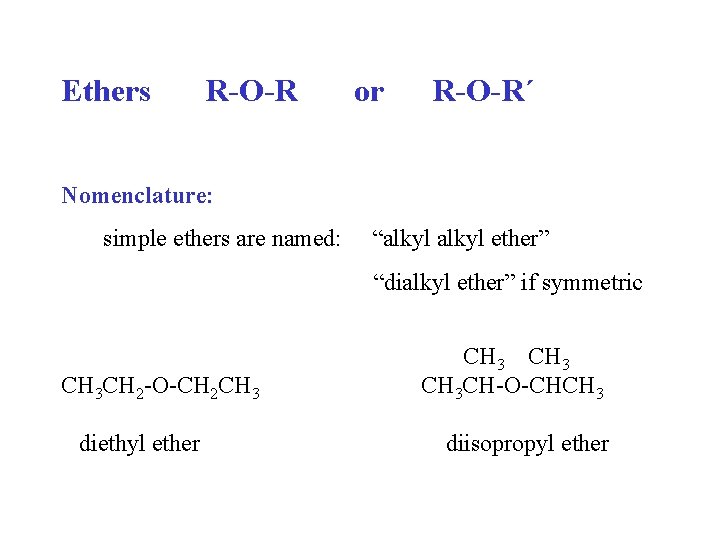
Ethers R-O-R or R-O-R´ Nomenclature: simple ethers are named: “alkyl ether” “dialkyl ether” if symmetric CH 3 CH 2 -O-CH 2 CH 3 diethyl ether CH 3 CH-O-CHCH 3 diisopropyl ether

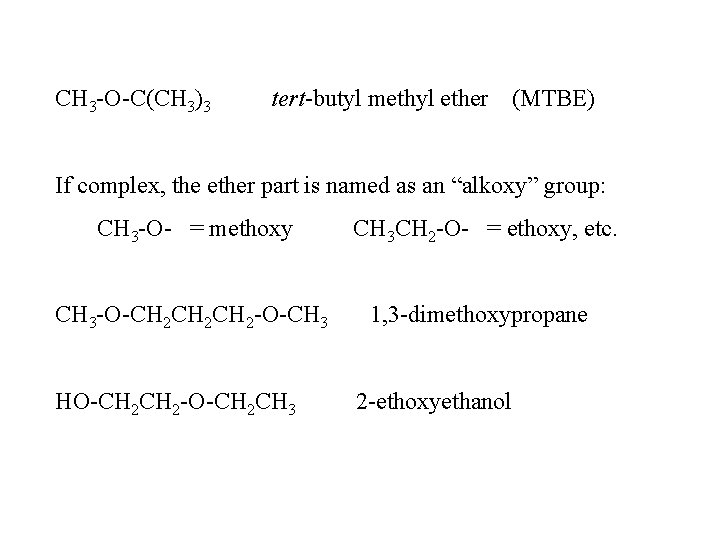
CH 3 -O-C(CH 3)3 tert-butyl methyl ether (MTBE) If complex, the ether part is named as an “alkoxy” group: CH 3 -O- = methoxy CH 3 CH 2 -O- = ethoxy, etc. CH 3 -O-CH 2 CH 2 -O-CH 3 1, 3 -dimethoxypropane HO-CH 2 -O-CH 2 CH 3 2 -ethoxyethanol

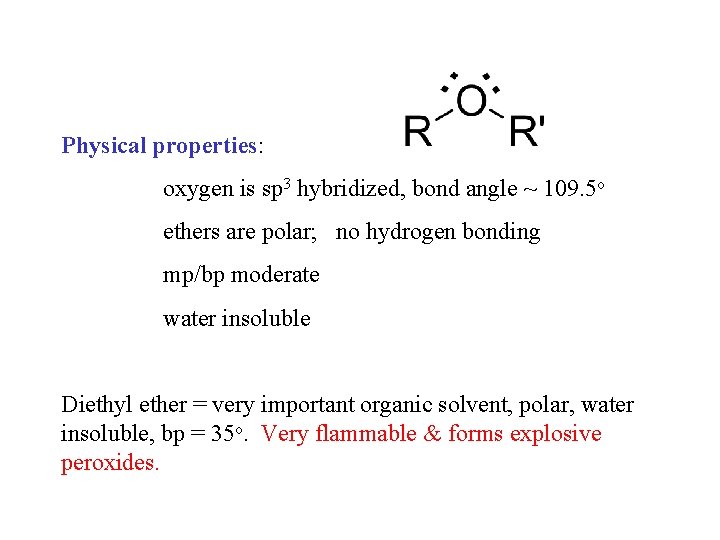
Physical properties: oxygen is sp 3 hybridized, bond angle ~ 109. 5 o ethers are polar; no hydrogen bonding mp/bp moderate water insoluble Diethyl ether = very important organic solvent, polar, water insoluble, bp = 35 o. Very flammable & forms explosive peroxides.

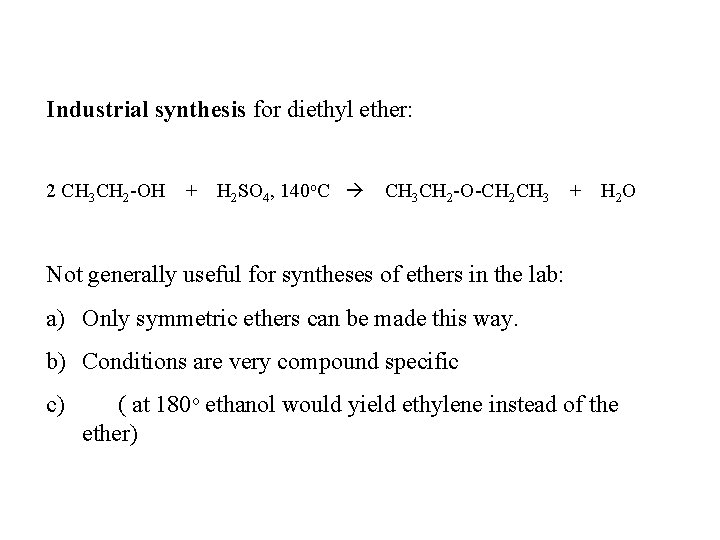
Industrial synthesis for diethyl ether: 2 CH 3 CH 2 -OH + H 2 SO 4, 140 o. C CH 3 CH 2 -O-CH 2 CH 3 + H 2 O Not generally useful for syntheses of ethers in the lab: a) Only symmetric ethers can be made this way. b) Conditions are very compound specific c) ( at 180 o ethanol would yield ethylene instead of the ether)

Synthesis of ethers 1. Williamson Synthesis. 2. R-O-Na+ + R´-X 3. R´-X should be CH 3 or 1 o 4. SN 2 mechanism R-O-R´ + Na. X

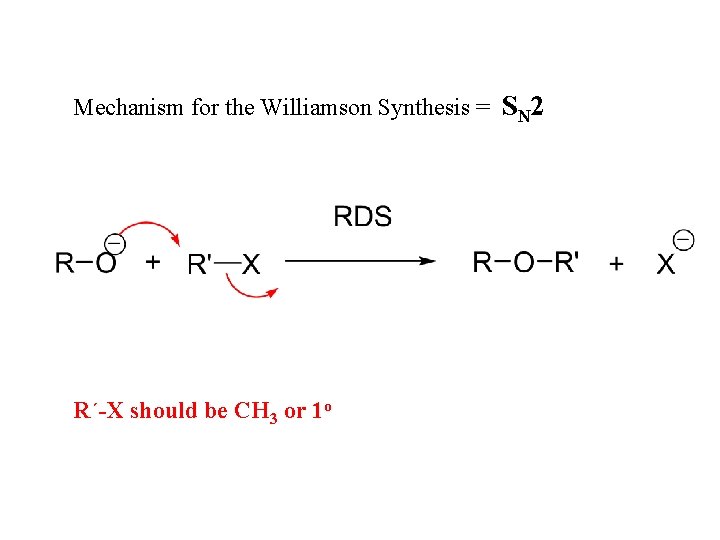
Mechanism for the Williamson Synthesis = SN 2 R´-X should be CH 3 or 1 o

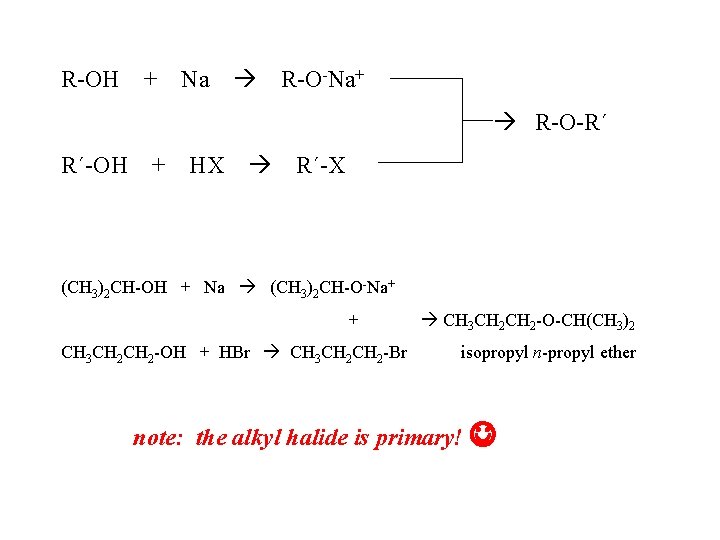
R-OH + Na R-O-Na+ R-O-R´ R´-OH + HX R´-X (CH 3)2 CH-OH + Na (CH 3)2 CH-O-Na+ + CH 3 CH 2 -OH + HBr CH 3 CH 2 CH 2 -O-CH(CH 3)2 isopropyl n-propyl ether note: the alkyl halide is primary!

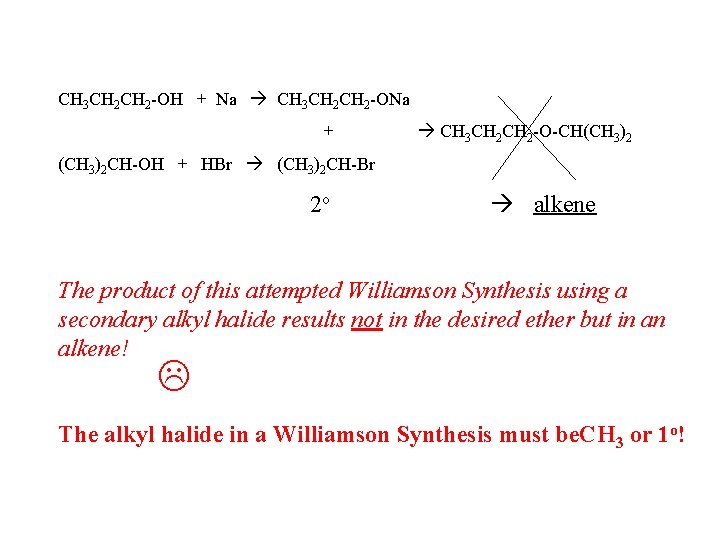
CH 3 CH 2 -OH + Na CH 3 CH 2 -ONa + CH 3 CH 2 -O-CH(CH 3)2 CH-OH + HBr (CH 3)2 CH-Br 2 o alkene The product of this attempted Williamson Synthesis using a secondary alkyl halide results not in the desired ether but in an alkene! The alkyl halide in a Williamson Synthesis must be. CH 3 or 1 o!

Synthesis of di-tert-butyl ether? CH 3 C-O-CCH 3 Cannot be made via the Williamson Synthesis. The alkyl halide would be 3 o. 2. Alkoxymercuration-demercuration 3. A way to make ethers that cannot be made via the Williamson 4. Synthesis. (later)

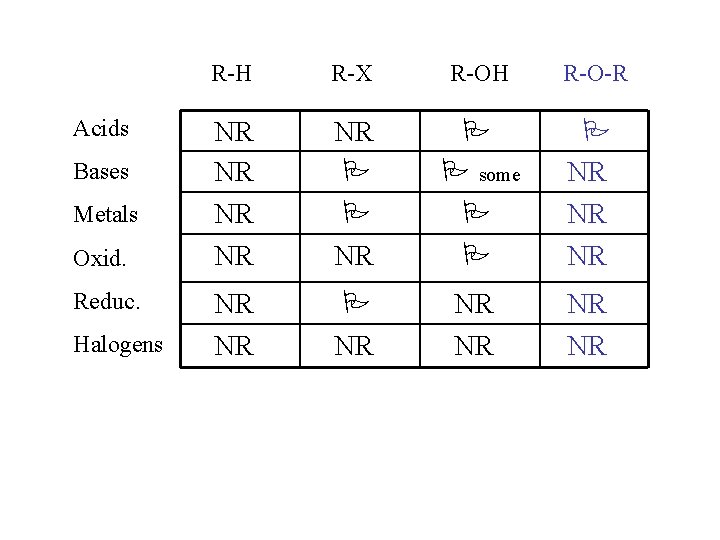
Acids Bases Metals Oxid. Reduc. Halogens R-H R-X R-OH R-O-R NR NR NR some NR NR NR

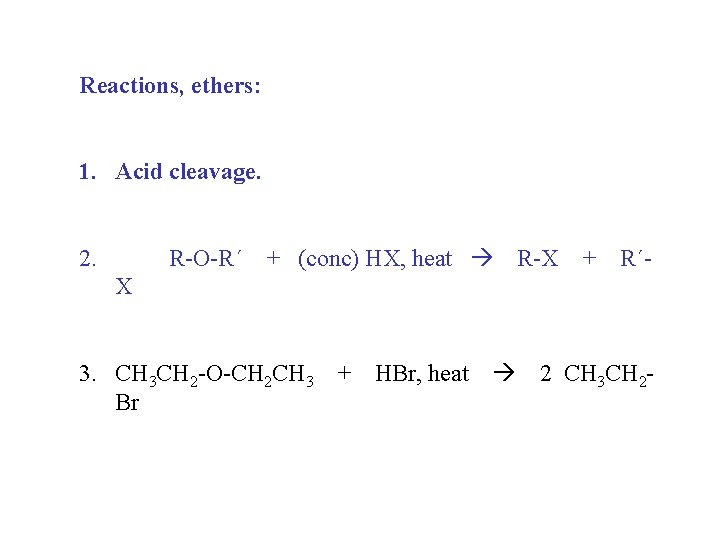
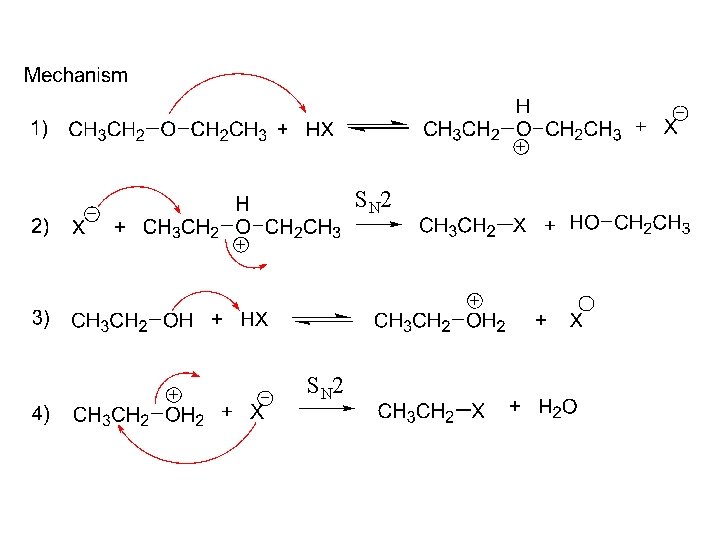
Reactions, ethers: 1. Acid cleavage. 2. R-O-R´ + (conc) HX, heat R-X + R´- X 3. CH 3 CH 2 -O-CH 2 CH 3 + HBr, heat 2 CH 3 CH 2 Br

S N 2

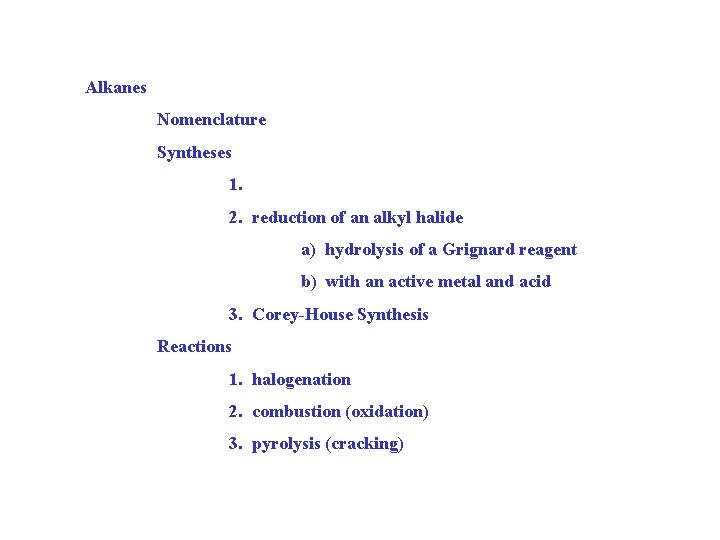
Alkanes Nomenclature Syntheses 1. 2. reduction of an alkyl halide a) hydrolysis of a Grignard reagent b) with an active metal and acid 3. Corey-House Synthesis Reactions 1. halogenation 2. combustion (oxidation) 3. pyrolysis (cracking)

Alkyl halides: nomenclature syntheses: 1. from alcohols a) HX b) PX 3 2. halogenation of certain alkanes 3. 4. 5. halide exchange for iodide reactions: 1. nucleophilic substitution 2. 3. formation of Grignard reagent 4. reduction

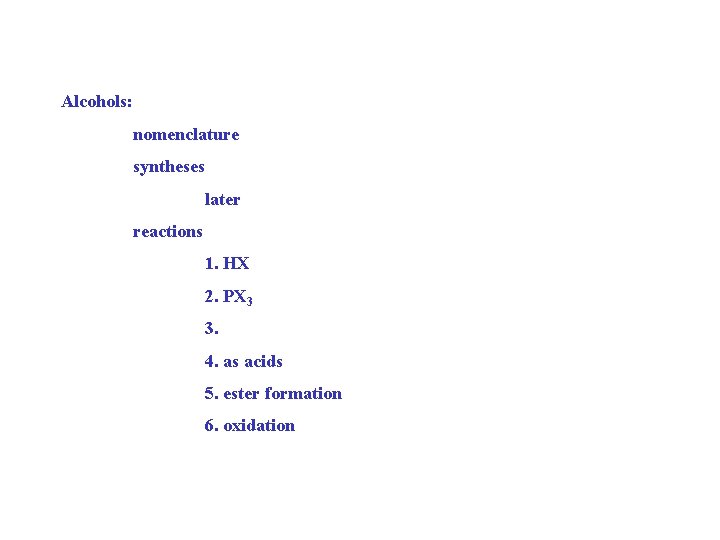
Alcohols: nomenclature syntheses later reactions 1. HX 2. PX 3 3. 4. as acids 5. ester formation 6. oxidation

Ethers nomenclature syntheses 1. Williamson Synthesis 2. reactions 1. acid cleavage

Mechanisms: Free radical substitution SN 2 SN 1 Memorize (all steps, curved arrow formalism, RDS) and know which reactions go by these mechanisms!