ETHERS Organic compounds which contain a divalent oxygen
- Slides: 13

ETHERS Organic compounds which contain a divalent oxygen atom bonded to two alkyl or aryl groups General formula R – O – R Classification On the basis of same or different alkyl or aryl group Symmetrical (simple) Ethers: When two alkyl groups joined to '– O –' are same CH 3 – O – CH 3 Dimethyl ether C 2 H 5 – O – C 2 H 5 Diethyl ether Unsymmetrical (mixed) Ethers: When two alkyl groups joined to – O – are different.

CH 3 – O – C 2 H 5 – O –C 3 H 7 Ethyl methyl ether Ethyl propyl ether B. On the basis of alkyl or aryl group attached on either side of oxygen Aliphatic Ethers: Ethereal oxygen is attached to both alkyl group CH 3 – O – CH 3 C 2 H 5 – O – C 2 H 5 Dimethyl ether Diethyl ether Aromatic Ethers: Ethereal oxygen is attached to both aryl group or one aryl group Methyl phenyl ether Diphenyl ether

Nomenclature CH 3 – O – C 2 H 5 C 2 H 5 – O – C 2 H 5 CH 3 – O – CH – (CH 3)2 Dimethyl ether Ethyl methyl ether Diethyl ether Methyl isopropyl ether Methyl phenyl ether Diphenyl ether

Isomerism (i) Chain isomerism C 2 H 5 – O – C 2 H 5 Diethyl ether (i) Functional isomerism: Ether CH 3 – O – CH 3 Dimethyl ether CH 3 – O – C 2 H 5 Ethyl methyl ether CH 3 – O –CH 2 CH 3 Methyl propyl ether Alcohol C 2 H 5 OH Ethyl alcohol C 3 H 7 OH Propyl alcohol

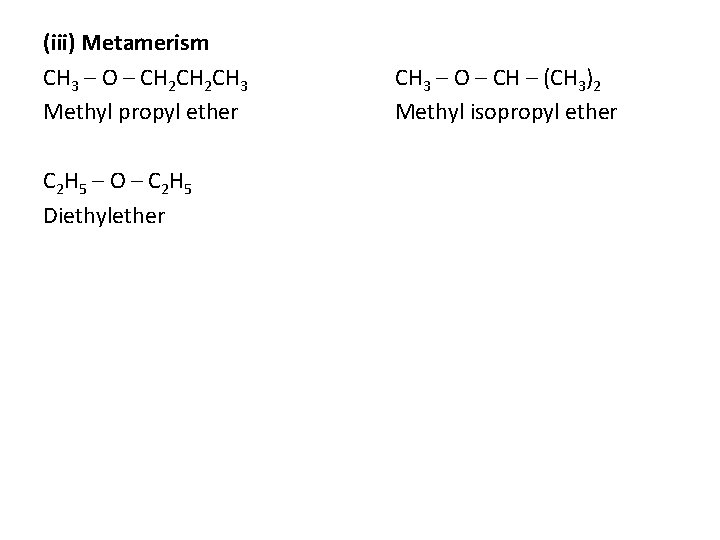
(iii) Metamerism CH 3 – O – CH 2 CH 3 Methyl propyl ether C 2 H 5 – O – C 2 H 5 Diethylether CH 3 – O – CH – (CH 3)2 Methyl isopropyl ether

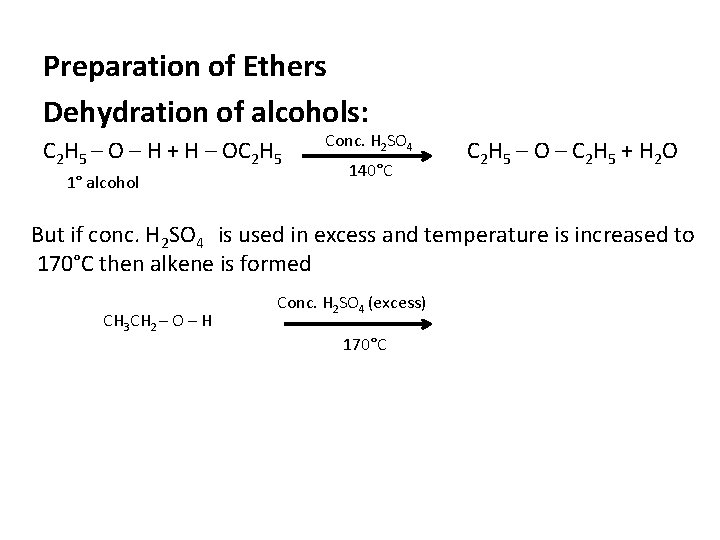
Preparation of Ethers Dehydration of alcohols: C 2 H 5 – O – H + H – OC 2 H 5 1° alcohol Conc. H 2 SO 4 140°C C 2 H 5 – O – C 2 H 5 + H 2 O But if conc. H 2 SO 4 is used in excess and temperature is increased to 170°C then alkene is formed CH 3 CH 2 – O – H Conc. H 2 SO 4 (excess) 170°C

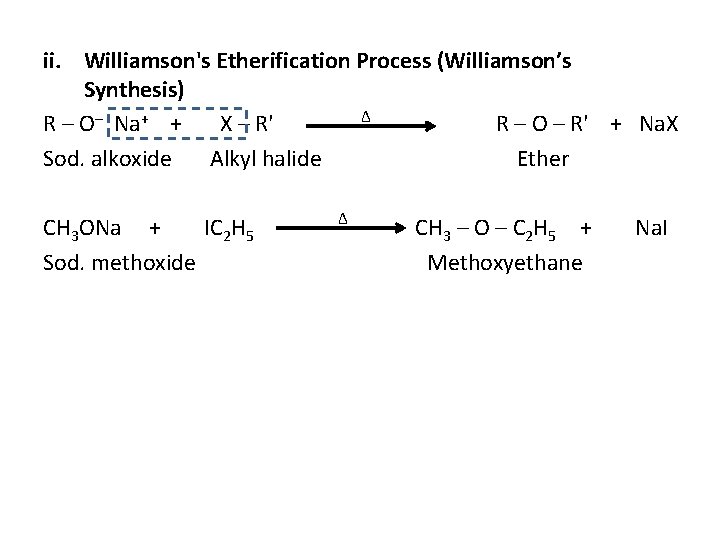
ii. Williamson's Etherification Process (Williamson’s Synthesis) ∆ R – O– Na+ + X – R' R – O – R' + Na. X Sod. alkoxide Alkyl halide Ether CH 3 ONa + IC 2 H 5 Sod. methoxide ∆ CH 3 – O – C 2 H 5 + Methoxyethane Na. I

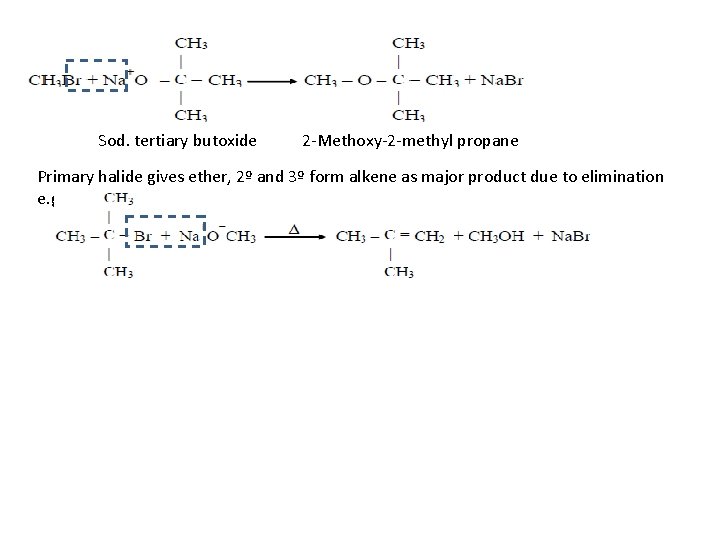
Sod. tertiary butoxide 2 -Methoxy-2 -methyl propane Primary halide gives ether, 2º and 3º form alkene as major product due to elimination e. g.

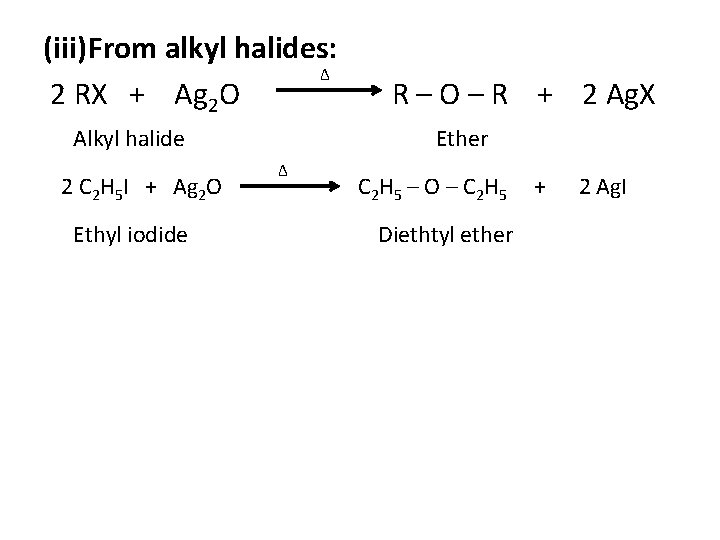
(iii)From alkyl halides: ∆ 2 RX + Ag 2 O Alkyl halide 2 C 2 H 5 I + Ag 2 O Ethyl iodide R – O – R + 2 Ag. X Ether ∆ C 2 H 5 – O – C 2 H 5 Diethtyl ether + 2 Ag. I

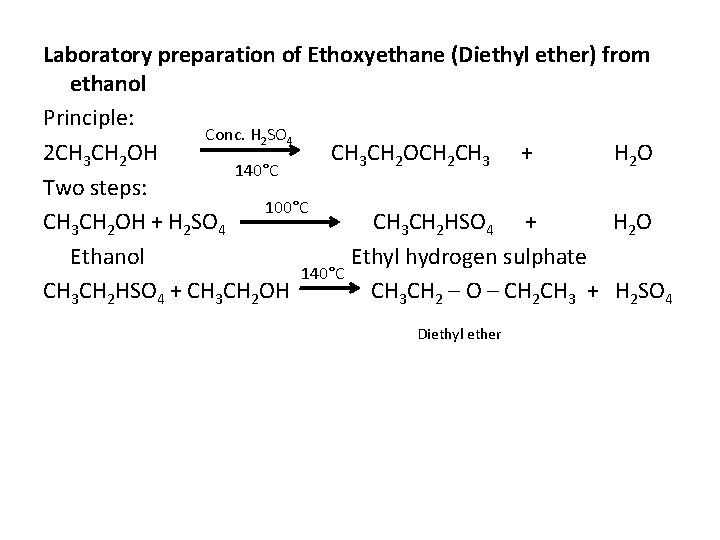
Laboratory preparation of Ethoxyethane (Diethyl ether) from ethanol Principle: Conc. H 2 SO 4 2 CH 3 CH 2 OH CH 3 CH 2 OCH 2 CH 3 + H 2 O 140°C Two steps: 100°C CH 3 CH 2 OH + H 2 SO 4 CH 3 CH 2 HSO 4 + H 2 O Ethanol Ethyl hydrogen sulphate 140°C CH 3 CH 2 HSO 4 + CH 3 CH 2 OH CH 3 CH 2 – O – CH 2 CH 3 + H 2 SO 4 Diethyl ether

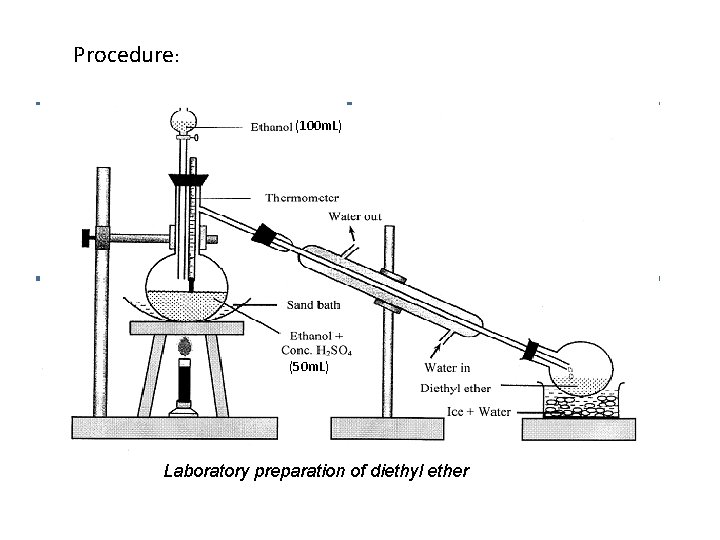
Procedure: (100 m. L) (50 m. L) Laboratory preparation of diethyl ether

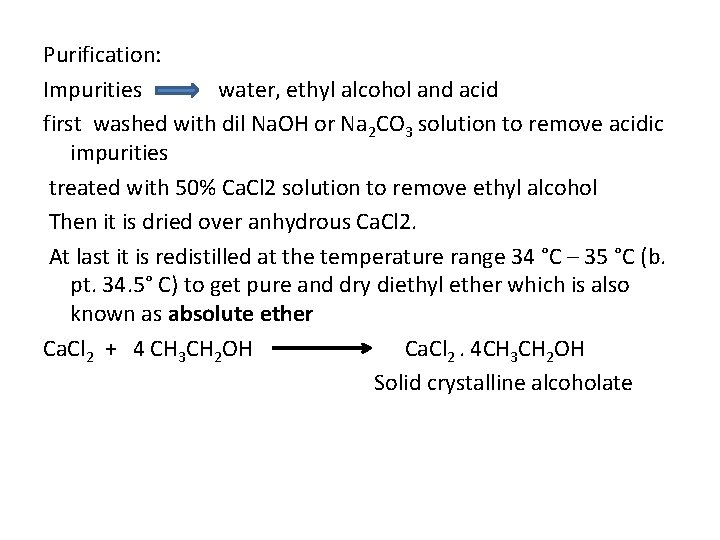
Purification: Impurities water, ethyl alcohol and acid first washed with dil Na. OH or Na 2 CO 3 solution to remove acidic impurities treated with 50% Ca. Cl 2 solution to remove ethyl alcohol Then it is dried over anhydrous Ca. Cl 2. At last it is redistilled at the temperature range 34 °C – 35 °C (b. pt. 34. 5° C) to get pure and dry diethyl ether which is also known as absolute ether Ca. Cl 2 + 4 CH 3 CH 2 OH Ca. Cl 2. 4 CH 3 CH 2 OH Solid crystalline alcoholate

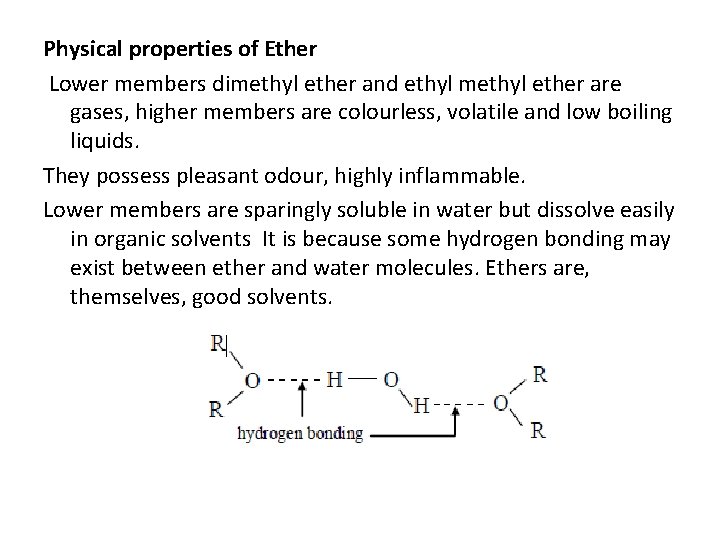
Physical properties of Ether Lower members dimethyl ether and ethyl methyl ether are gases, higher members are colourless, volatile and low boiling liquids. They possess pleasant odour, highly inflammable. Lower members are sparingly soluble in water but dissolve easily in organic solvents It is because some hydrogen bonding may exist between ether and water molecules. Ethers are, themselves, good solvents.