Ethanoldichromate titration Acidified potassium dichromate can be used

Ethanol-dichromate titration

Acidified potassium dichromate can be used to oxidise ethanol to ethanoic acid. These days instruments are used to quickly determine the concentration of ethanol in blood, but at one time blood alcohol levels (for drink-driving convictions) were determined by titration. The ethanol was separated from the blood sample and reacted with the dichromate solution. Calculate the concentration of ethanol contained in a 10. 0 m. L sample of blood that reacted with 13. 7 m. L of 0. 0209 mol L– 1 solution of potassium dichromate, and hence determine whether the blood exceeded the legal limit of 80 mg of ethanol per 100 m. L of blood.

Step 1: Determine the amount of the known reagent (the dichromate) c(Cr 2 O 72–) = 0. 0209 mol L– 1 V(Cr 2 O 72–) = 13. 7 m. L = 13. 7 10– 3 L L n(Cr 2 O 72–) = c. V = = 2. 863 10– 4 mol

Step 2: Use the equation for the chemical reaction to determine the amount of ethanol present 3 2 2 O 72– + 16 H+ → 3 CH 3 COOH + 4 Cr 3+ + 11 H 2 O 3 CH 2 OH + 2 Cr Unknown on top Known on the bottom n(CH 3 CH 2 OH) = 2– n(Cr 2 O 7 ) n(CH 3 CH 2 OH) n(Cr 2 O 7 2–) = Rearrange to get the unknown on its own. 3 2 3 2. 863 10– 4 = mol 2 = 4. 295 10– 4 mol
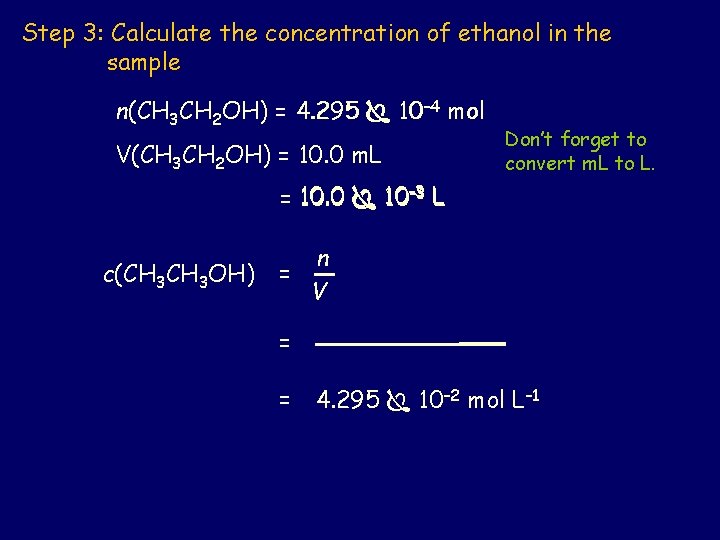
Step 3: Calculate the concentration of ethanol in the sample n(CH 3 CH 2 OH) = 4. 295 10– 4 mol V(CH 3 CH 2 OH) = 10. 0 m. L Don’t forget to convert m. L to L. = 10. 0 10– 3 L n c(CH 3 OH) = V = = 4. 295 10– 2 mol L– 1

Step 4: Convert the concentration of ethanol in the blood sample into mg per 100 m. L of blood c(CH 3 CH 2 OH) = 4. 295 10– 2 mol L– 1 M(CH 3 CH 2 OH) = 46. 0 g mol– 1 To convert concentration in mol L– 1 into g L– 1, multiply by the molar mass (in g mol– 1). c(CH 3 CH 2 OH) = = 1. 976 g L– 1 = 0. 198 g per 100 m. L = 198 mg per 100 m. L
- Slides: 6