Ethanol dimer Observation of three new conformers by

Ethanol dimer: Observation of three new conformers by broadband rotational spectroscopy D. Loru, I. Peña and M. E. Sanz Department of Chemistry King’s College London
Ethanol Dimer Motivation • To aid the assignment of complexes of odorants with ethanol • Archetypal system for the study of hydrogen bonded clusters Better understanding of the intermolecular forces at play
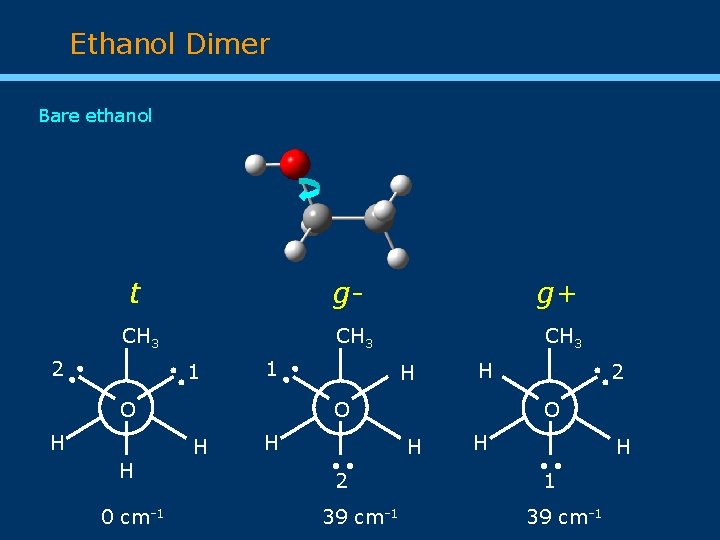
Ethanol Dimer Bare ethanol t g. CH 3 O H 0 cm-1 • H O H H H • • 1 H • • 2 39 cm-1 2 • • 1 CH 3 • • 2 • CH 3 g+ O H H • • 1 39 cm-1 H
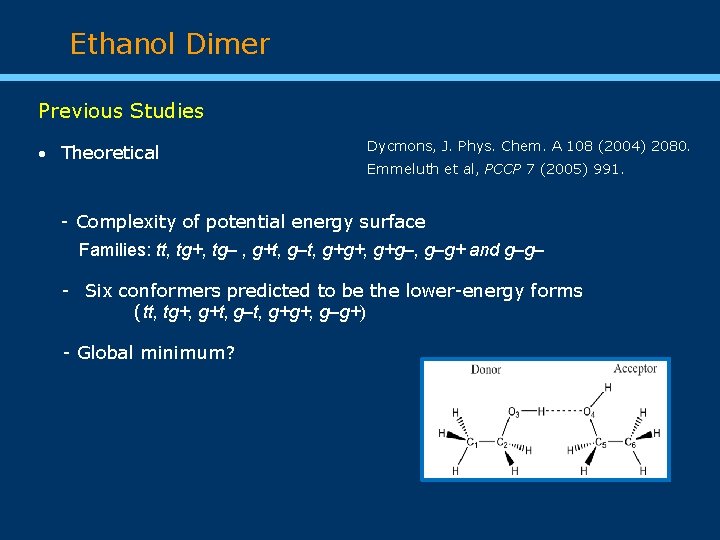
Ethanol Dimer Previous Studies Theoretical Dycmons, J. Phys. Chem. A 108 (2004) 2080. Emmeluth et al, PCCP 7 (2005) 991. - Complexity of potential energy surface Families: tt, tg+, tg‒ , g+t, g‒t, g+g+, g+g‒, g‒g+ and g‒g‒ - Six conformers predicted to be the lower-energy forms (tt, tg+, g+t, g‒t, g+g+, g‒g+) - Global minimum?
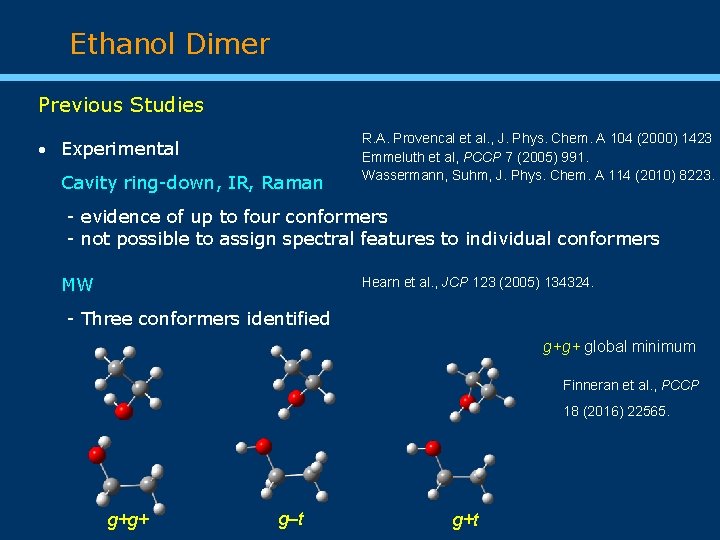
Ethanol Dimer Previous Studies Experimental Cavity ring-down, IR, Raman R. A. Provencal et al. , J. Phys. Chem. A 104 (2000) 1423 Emmeluth et al, PCCP 7 (2005) 991. Wassermann, Suhm, J. Phys. Chem. A 114 (2010) 8223. - evidence of up to four conformers - not possible to assign spectral features to individual conformers MW Hearn et al. , JCP 123 (2005) 134324. - Three conformers identified g+g+ global minimum Finneran et al. , PCCP 18 (2016) 22565. g+g+ g‒t g+t
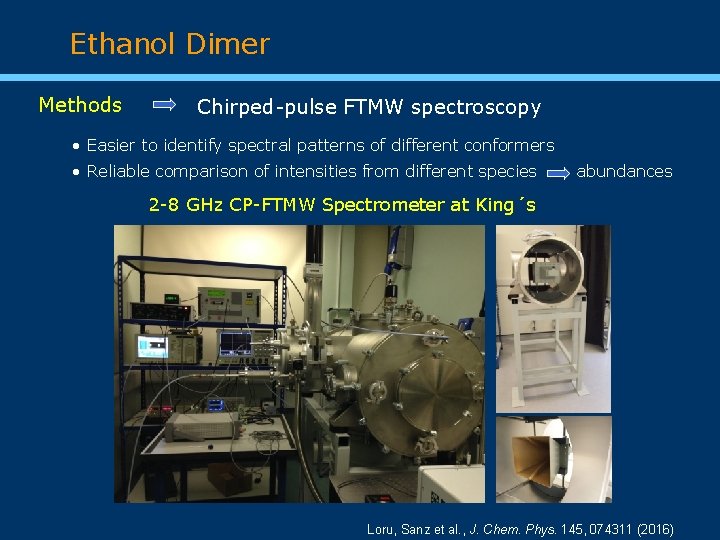
Ethanol Dimer Methods Chirped-pulse FTMW spectroscopy • Easier to identify spectral patterns of different conformers • Reliable comparison of intensities from different species abundances 2 -8 GHz CP-FTMW Spectrometer at King´s Loru, Sanz et al. , J. Chem. Phys. 145, 074311 (2016)
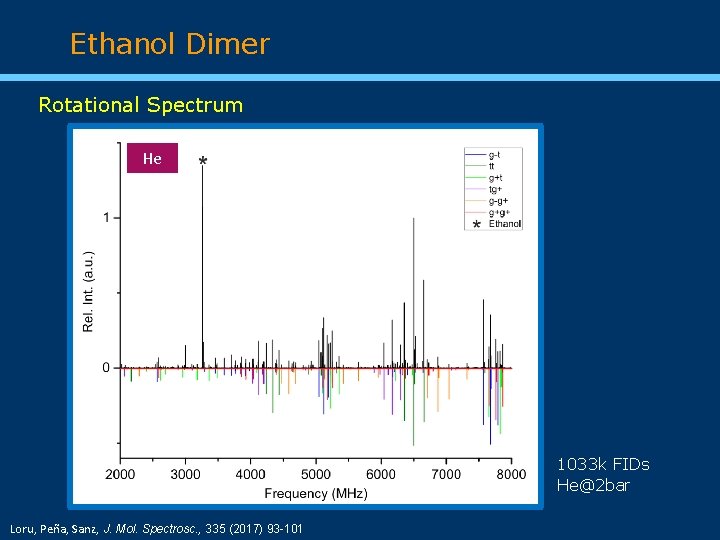
Ethanol Dimer Rotational Spectrum He 1033 k FIDs He@2 bar Loru, Peña, Sanz, J. Mol. Spectrosc. , 335 (2017) 93 -101
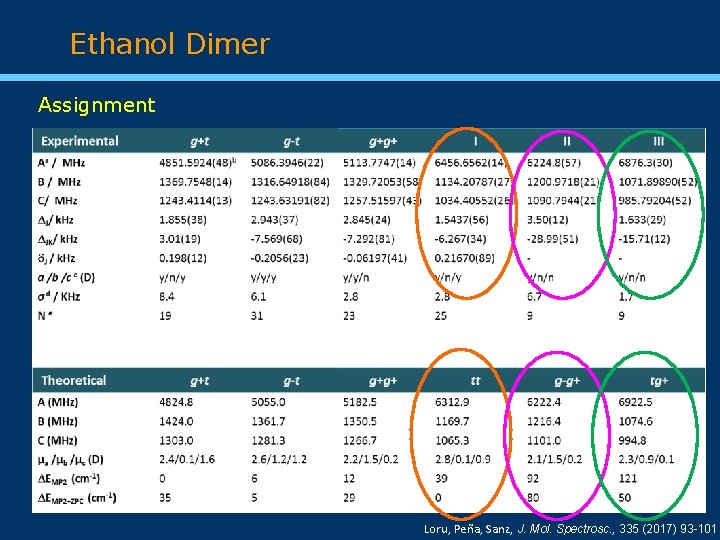
Ethanol Dimer Assignment Loru, Peña, Sanz, J. Mol. Spectrosc. , 335 (2017) 93 -101
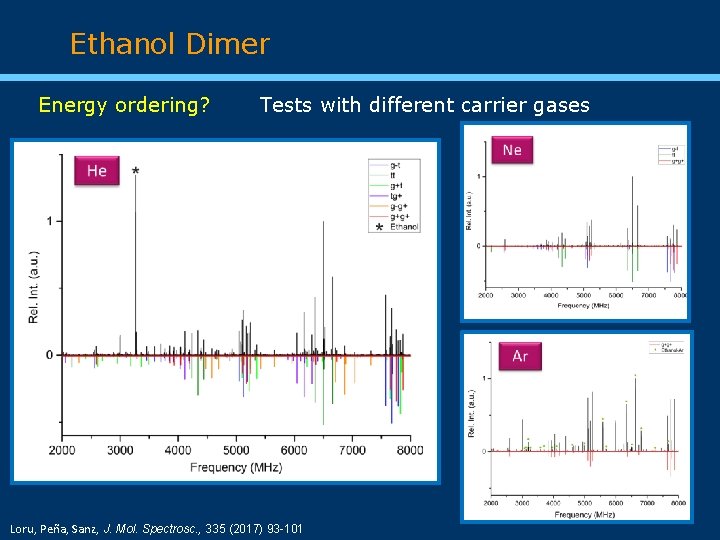
Ethanol Dimer Energy ordering? Tests with different carrier gases Loru, Peña, Sanz, J. Mol. Spectrosc. , 335 (2017) 93 -101
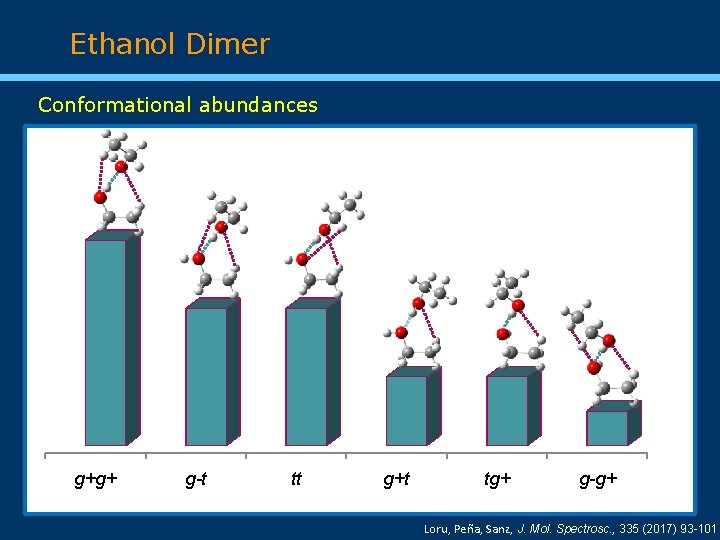
Ethanol Dimer Conformational abundances g+g+ g-t tt g+t tg+ g-g+ Loru, Peña, Sanz, J. Mol. Spectrosc. , 335 (2017) 93 -101
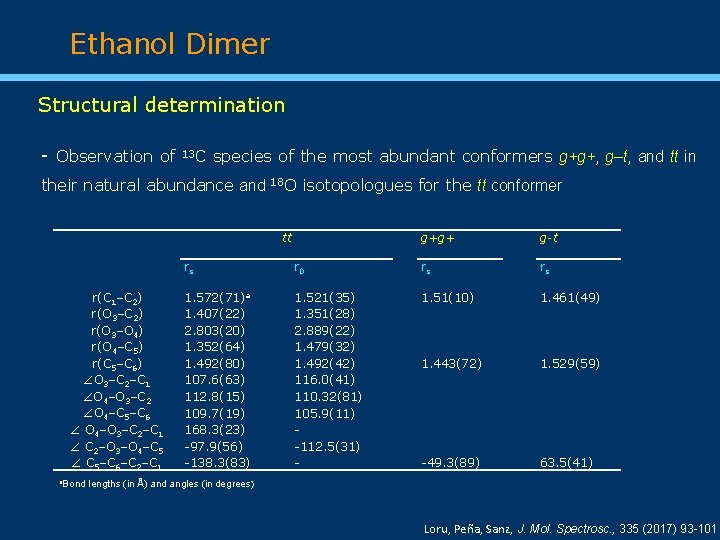
Ethanol Dimer Structural determination - Observation of 13 C species of the most abundant conformers g+g+, g‒t, and tt in their natural abundance and 18 O isotopologues for the tt conformer tt r(C 1–C 2) r(O 3–O 4) r(O 4–C 5) r(C 5–C 6) O 3–C 2–C 1 O 4–O 3–C 2 O 4–C 5–C 6 O 4–O 3–C 2–C 1 C 2–O 3–O 4–C 5 C 5–C 6–C 2–C 1 a. Bond g+g+ g-t rs r 0 rs rs 1. 572(71)a 1. 407(22) 2. 803(20) 1. 352(64) 1. 492(80) 107. 6(63) 112. 8(15) 109. 7(19) 168. 3(23) -97. 9(56) -138. 3(83) 1. 521(35) 1. 351(28) 2. 889(22) 1. 479(32) 1. 492(42) 116. 0(41) 110. 32(81) 105. 9(11) -112. 5(31) - 1. 51(10) 1. 461(49) 1. 443(72) 1. 529(59) -49. 3(89) 63. 5(41) lengths (in Å) and angles (in degrees) Loru, Peña, Sanz, J. Mol. Spectrosc. , 335 (2017) 93 -101
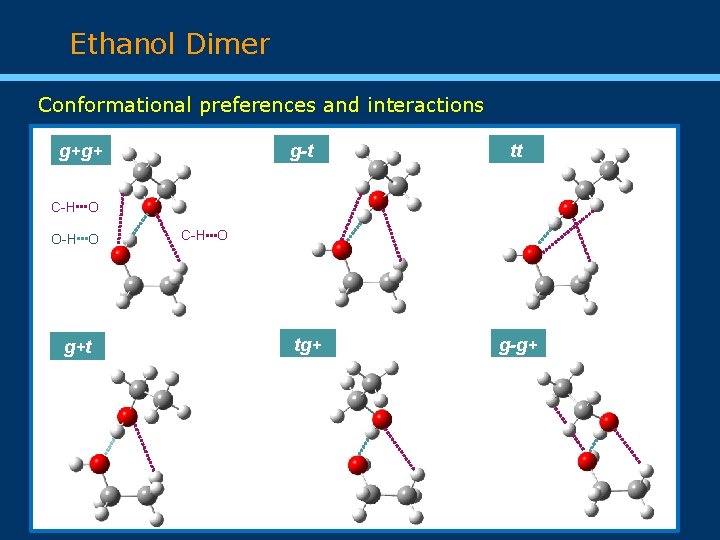
Ethanol Dimer Conformational preferences and interactions g+g+ g-t tt C-H O O-H O g+t C-H O tg+ g-g+

Conclusions Three new conformers (tt, tg+, and g-g+) identified together with the three previously characterized. Several isotopologues in natural abundance observed for the most abundant conformers (g+g+, g-t, and tt). Substitution and effective (only for tt) structures derived. Use of different carrier gases to establish the conformational energy ordering of the six observed conformers, confirming the homochiral conformer g+g+ as the lowest energy form. Intermolecular interactions involving classical O-H···O hydrogen bonds and weak C-H···O and dispersion interactions determine the relative stability of the observed conformations.

AKNOWLEDGEMENTS Dr. M. E. Sanz D. Loru E Burevschi N Jarman J Tang FUNDING
- Slides: 14