Estetrol the fourth natural human Estrogen Prof J













- Slides: 61

Estetrol, the fourth natural human Estrogen Prof. J. M. Foidart University of Liege, and Estetra Belgium VVOG, Jaar congres 18 -19 oktober 2012 Bredene


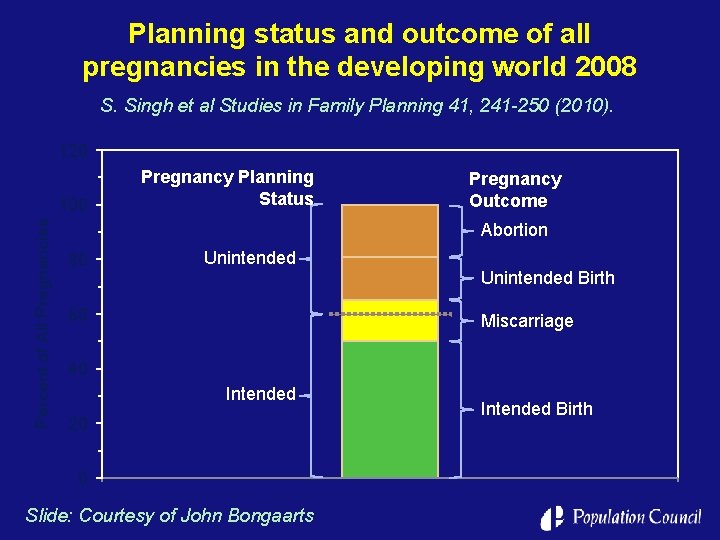
Planning status and outcome of all pregnancies in the developing world 2008 S. Singh et al Studies in Family Planning 41, 241 -250 (2010). 120 Percent of All Pregnancies 100 Pregnancy Planning Status Pregnancy Outcome Abortion 80 Unintended Birth 60 Miscarriage 40 Intended 20 0 Slide: Courtesy of John Bongaarts Intended Birth

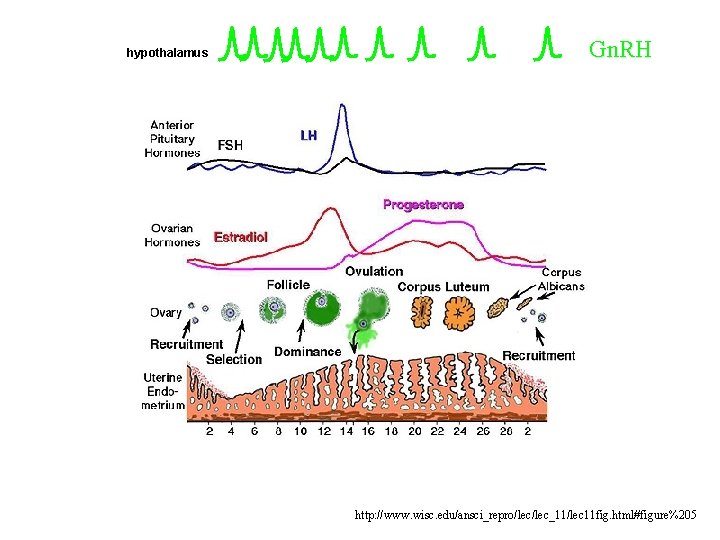
hypothalamus Gn. RH http: //www. wisc. edu/ansci_repro/lec_11/lec 11 fig. html#figure%205

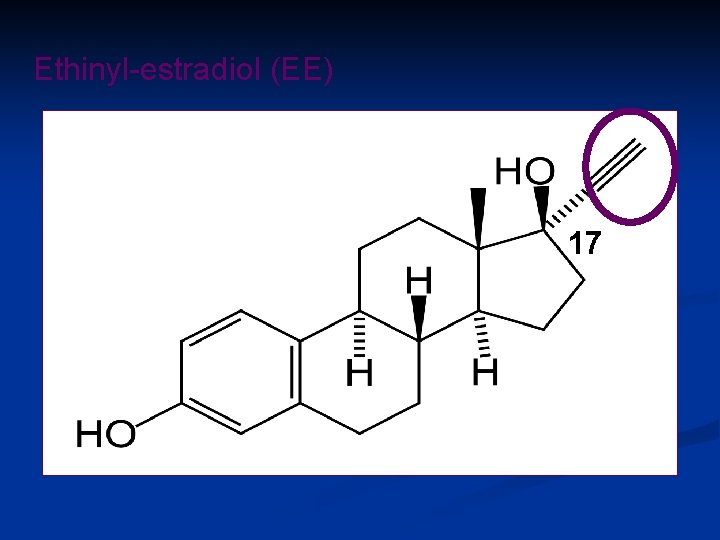
Why an estrogen in the pill ? • Major reason – To improve the menstrual bleeding pattern • Additional effects – To assist the progestin in inhibiting ovulation and suppressing FSH – To prevent estrogen deficiency (vaginal atrophy, decreased bone formation), • Most combined oral contraceptives contain ethinylestradiol (EE) Are there reasons to replace EE?

Ethinyl-estradiol (EE) 17

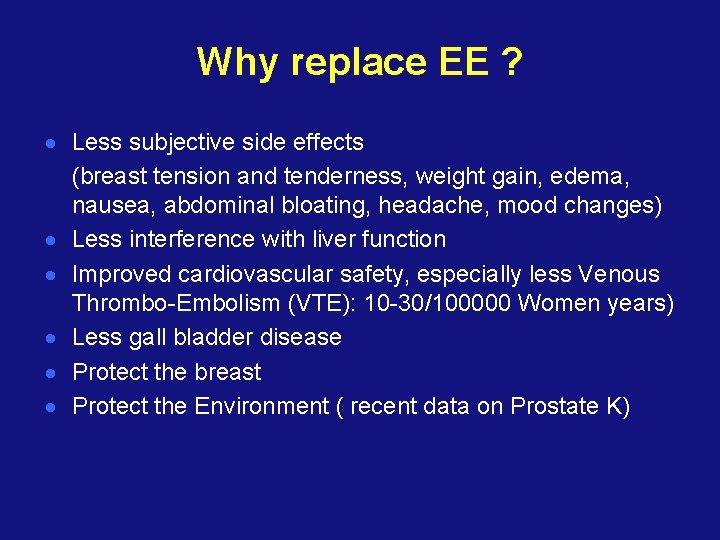
Why replace EE ? · Less subjective side effects (breast tension and tenderness, weight gain, edema, nausea, abdominal bloating, headache, mood changes) · Less interference with liver function · Improved cardiovascular safety, especially less Venous Thrombo-Embolism (VTE): 10 -30/100000 Women years) · Less gall bladder disease · Protect the breast · Protect the Environment ( recent data on Prostate K)

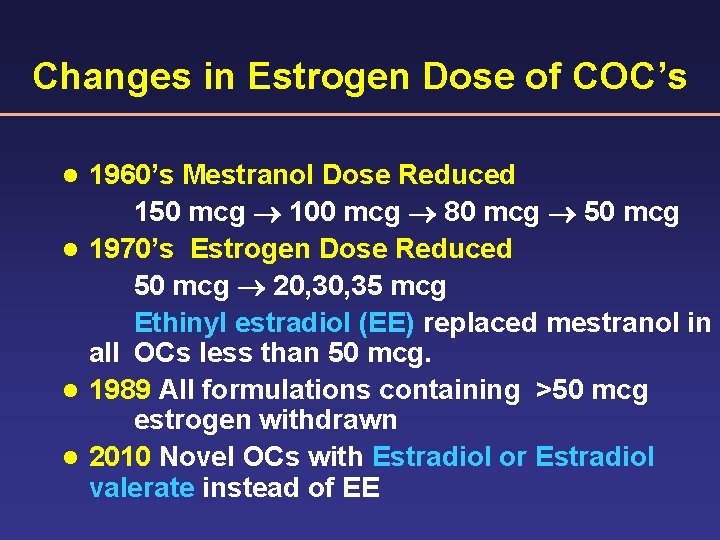
Changes in Estrogen Dose of COC’s 1960’s Mestranol Dose Reduced 150 mcg 100 mcg 80 mcg 50 mcg l 1970’s Estrogen Dose Reduced 50 mcg 20, 35 mcg Ethinyl estradiol (EE) replaced mestranol in all OCs less than 50 mcg. l 1989 All formulations containing >50 mcg estrogen withdrawn l 2010 Novel OCs with Estradiol or Estradiol valerate instead of EE l

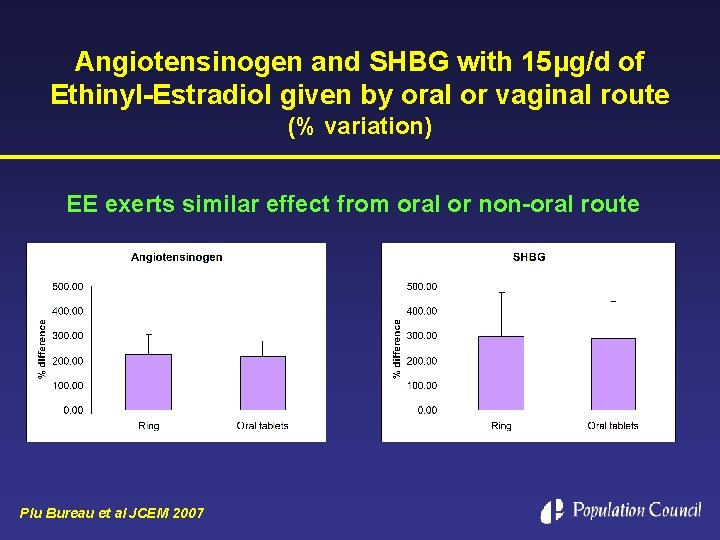
Angiotensinogen and SHBG with 15µg/d of Ethinyl-Estradiol given by oral or vaginal route (% variation) EE exerts similar effect from oral or non-oral route Plu Bureau et al JCEM 2007

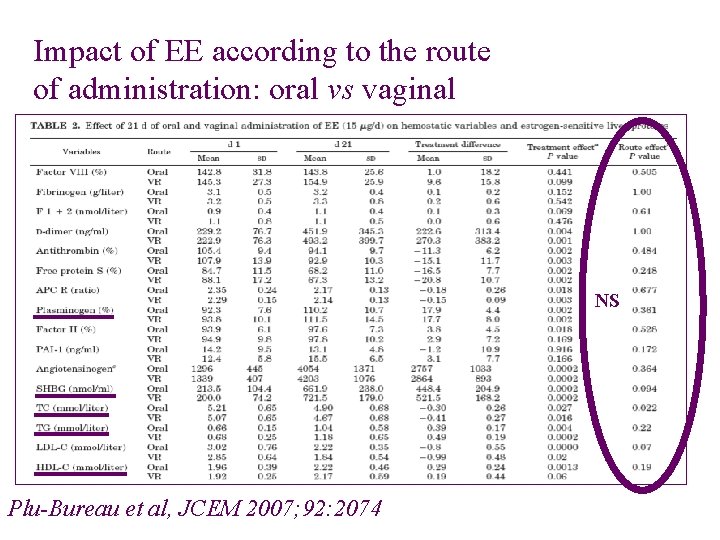
Impact of EE according to the route of administration: oral vs vaginal NS Plu-Bureau et al, JCEM 2007; 92: 2074

BMJ October 2011

LNG + EE: 2. 9 (2. 2 -3. 8) Gestodene + EE: 6. 2 (5. 6 -7. 0) Desogestrel + EE: 6. 6 (5. 6 - 7. 8) Drospirenone + EE: 6. 4 (5. 4 -7. 5) As compared to LNG + EE: DSG: 2. 2 GES + EE: 2. 1 Drospirenone +EE: 2. 1


Disadvantages of EE according to an E 2/Nomac patent • Ethinyloestradiol has a very strong impact on liver function – disorders in the synthesis of clotting factors – abnomalies in the lipid profile – problematic in at-risk women (women suffering from circulatory disorders, women in the perimenopause, women who smoke) • The deleterious effect of ethinyloestradiol can be further increased by the progestative fraction on account of a residual androgenic activity which is often present • Replace EE with the hormone secreted by the ovaries, 17 beta-oestradiol (E 2), which is much less toxic than EE

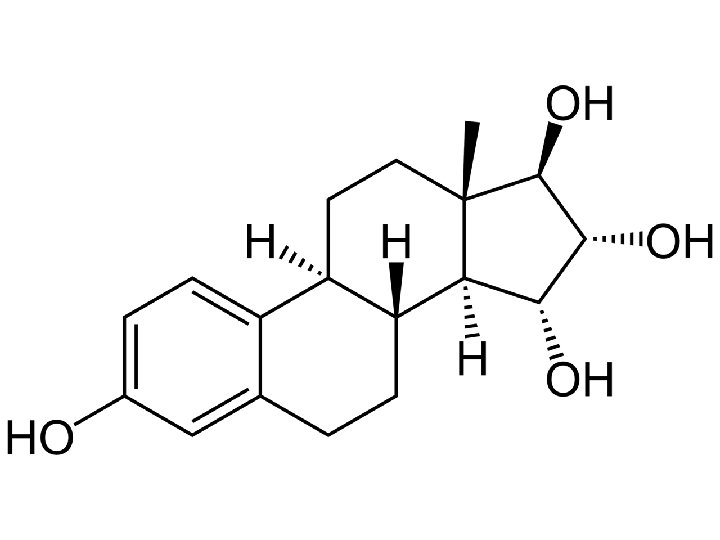
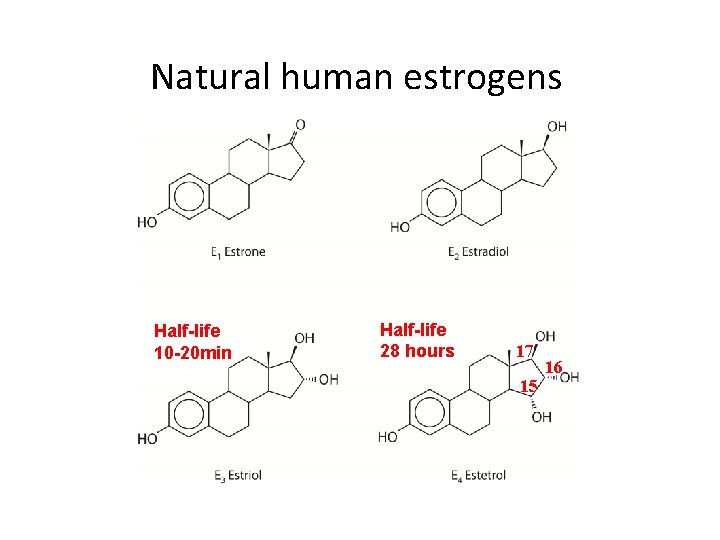
Natural human estrogens Half-life 10 -20 min Half-life 28 hours 17 15 16

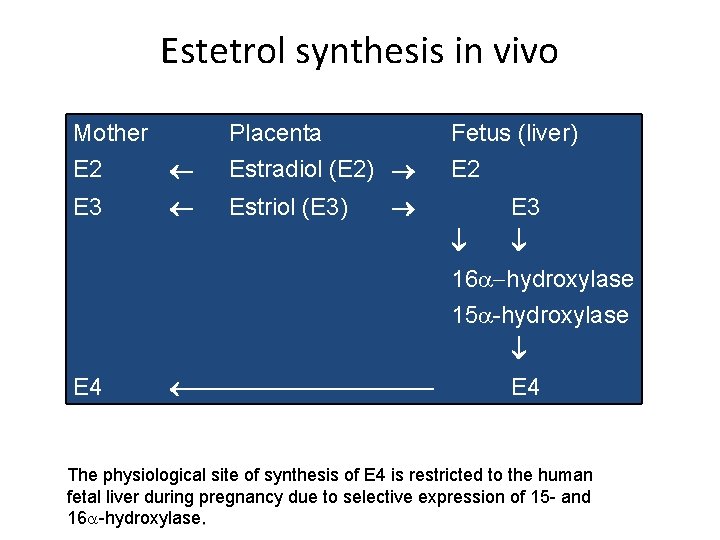
Estetrol synthesis in vivo Mother E 2 E 3 Placenta Estradiol (E 2) Estriol (E 3) Fetus (liver) E 2 E 3 16 a-hydroxylase 15 a-hydroxylase E 4 The physiological site of synthesis of E 4 is restricted to the human fetal liver during pregnancy due to selective expression of 15 - and 16 a-hydroxylase.

Estetrol is human- and pregnancy-specific • Estetrol is detected in the human foetus and in pregnant women from 9 weeks gestation onwards. • The maternal mean plasma E 4 level at term of 1. 2 ng/ml is 7 -10 fold higher than that observed at 24 weeks of gestation. No diurnal variations are found. • E 4 levels, in fetal plasma at term, are 12 -fold higher than those in maternal plasma and no fetal arterial venous differences are identified. Tulchinsky D, et al. Plasma estetrol as an index of fetal well-being. J Clin Endocrinol Metab. 1975; 40 : 560 -7

Estetrol is human- and pregnancy-specific • Term fetal liver production is 3 mg/day • Term fetal exposure is comparable to 50 -60 mg daily oral treatment in early postmenopausal women (kinetic simulation) • Estetrol was not found during pregnancy in the following species: o Rat, mouse, o Horse (pregnant mare) o Monkey (macaque)

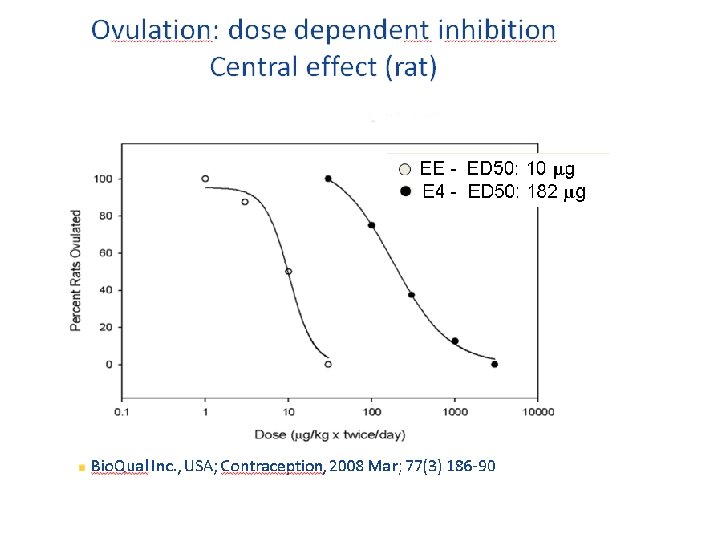
Why does the human fetus produce Estetrol ? • The human fetus expresses estrogen receptors early during development, but Estrogens do not exert strong estrogenic effects on the fetus (no feminisation of males, no major endometrial growth in females) • Hypothetical functions of E 4: – Maternal vasodilation to/in the uterus – Immunological protection of the fetus – Protection of the breast against E 2 – Fetal (positive) bone formation – Role in the initiation of labor – Brain neuroprotection

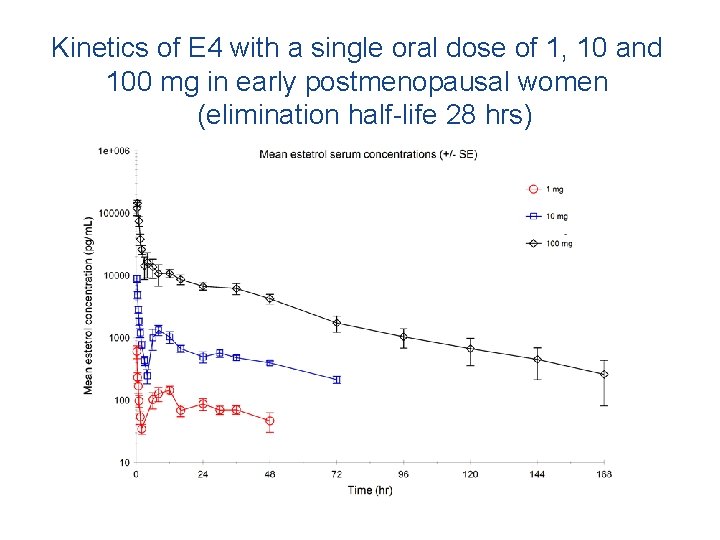
Kinetics of E 4 with a single oral dose of 1, 10 and 100 mg in early postmenopausal women (elimination half-life 28 hrs)

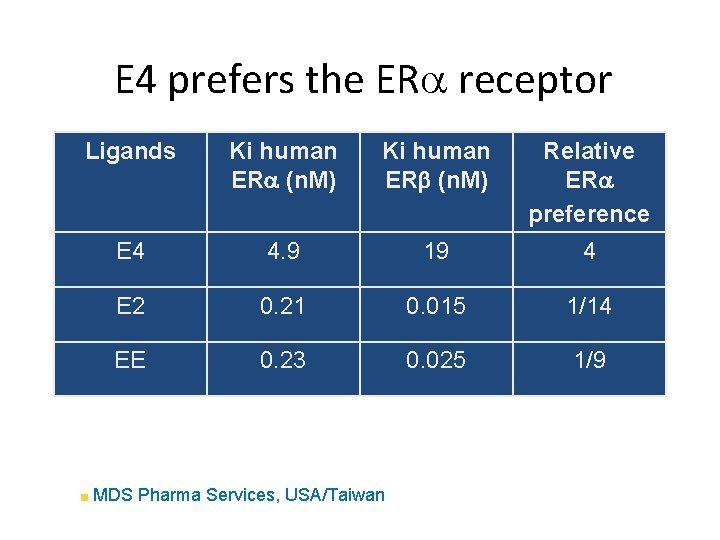
E 4 prefers the ERa receptor Ligands Ki human ERa (n. M) Ki human ERb (n. M) Relative ERa preference E 4 4. 9 19 4 E 2 0. 21 0. 015 1/14 EE 0. 23 0. 025 1/9 MDS Pharma Services, USA/Taiwan


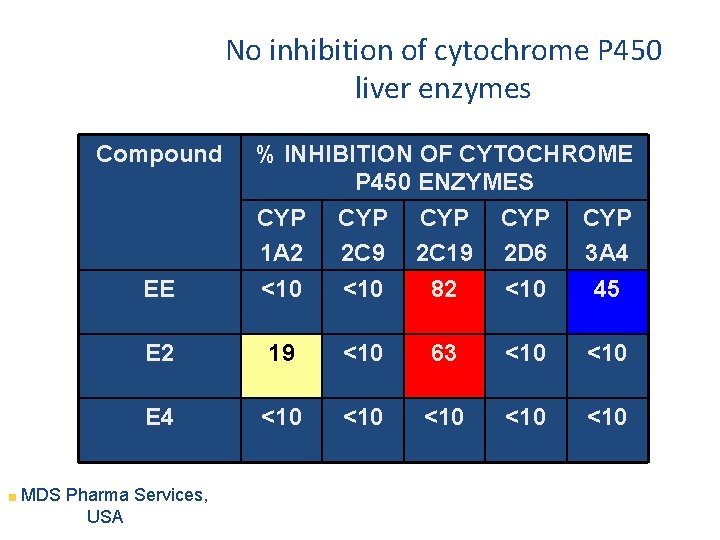
No inhibition of cytochrome P 450 liver enzymes Compound % INHIBITION OF CYTOCHROME P 450 ENZYMES CYP 1 A 2 CYP 2 C 9 CYP 2 C 19 CYP 2 D 6 CYP 3 A 4 EE <10 82 <10 45 E 2 19 <10 63 <10 E 4 <10 <10 <10 MDS Pharma Services, USA



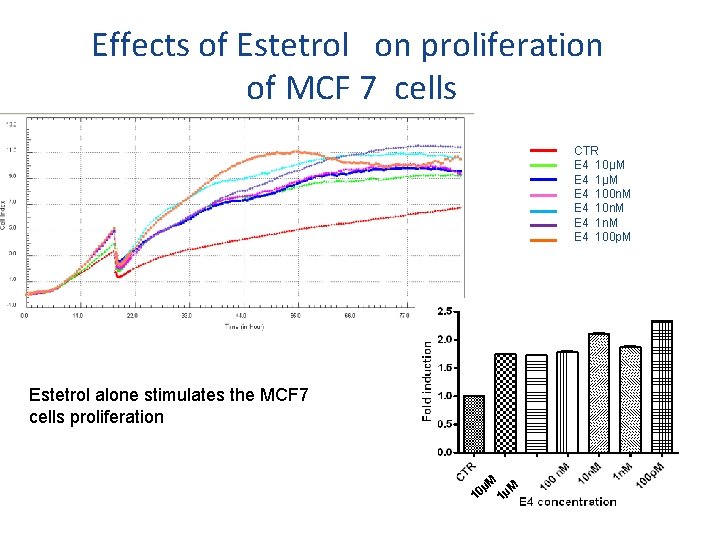
Effects of Estetrol on proliferation of MCF 7 cells CTR E 4 10µM E 4 100 n. M E 4 1 n. M E 4 100 p. M Estetrol alone stimulates the MCF 7 cells proliferation µM µM 0 1 1

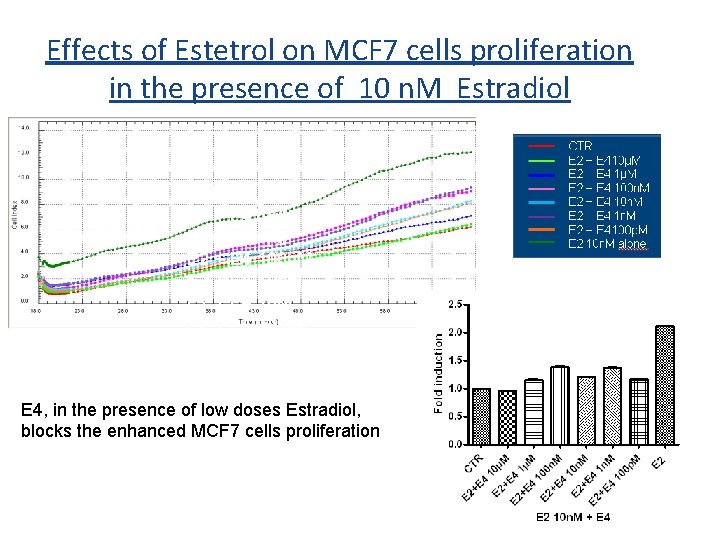
Effects of Estetrol on MCF 7 cells proliferation in the presence of 10 n. M Estradiol CTR E 2 + E 410µM E 2 + E 4 100 n. M E 2 + E 4 1 n. M E 2 + E 4100 p. M E 2 10 n. M E 4, in the presence of low doses Estradiol, blocks the enhanced MCF 7 cells proliferation

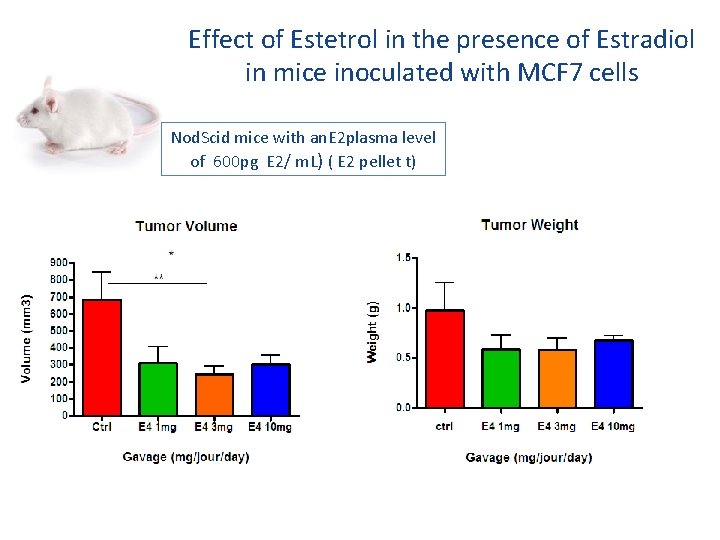
Effect of Estetrol in the presence of Estradiol in mice inoculated with MCF 7 cells Nod. Scid mice with an. E 2 plasma level of 600 pg E 2/ m. L) ( E 2 pellet t)

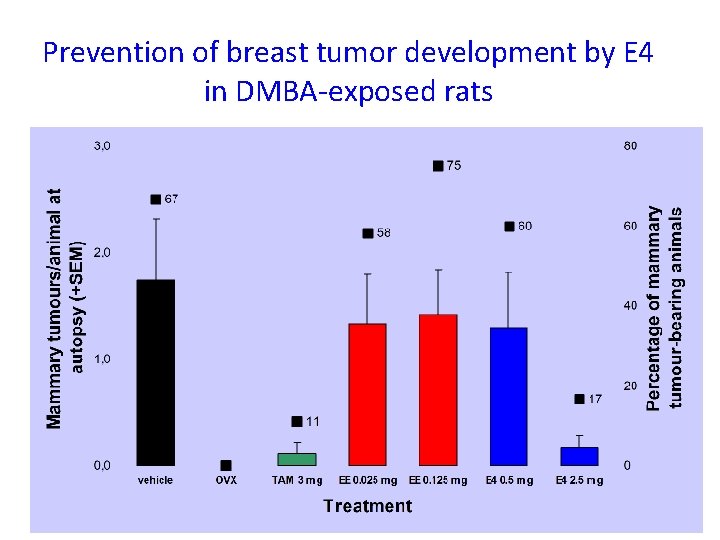
Prevention of breast tumor development by E 4 in DMBA-exposed rats

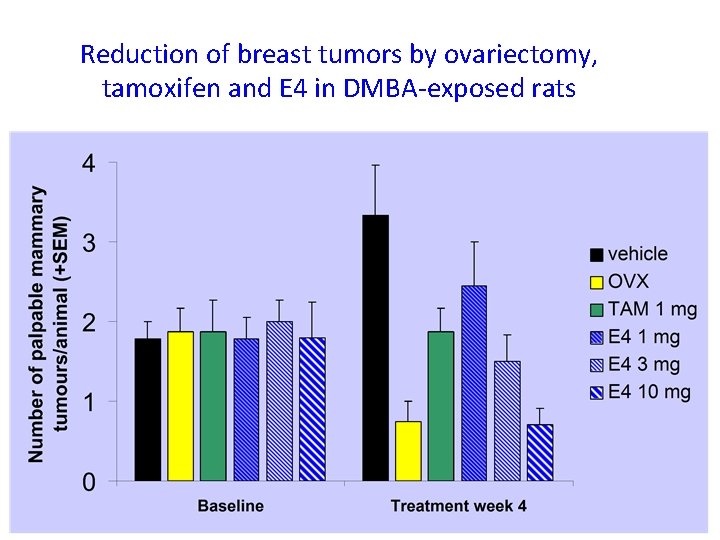
Reduction of breast tumors by ovariectomy, tamoxifen and E 4 in DMBA-exposed rats

Pharmacological effects of Estetrol (E 4) n n n E 4 is orally bioavailable with a long half life E 4 is an estrogen agonist for: n Bone n Brain (hot flush model, inhibition of ovulation) n Vagina n Uterus n Endometrium (proliferation) E 4 is an estrogen antagonist for the breast in the presence of E 2

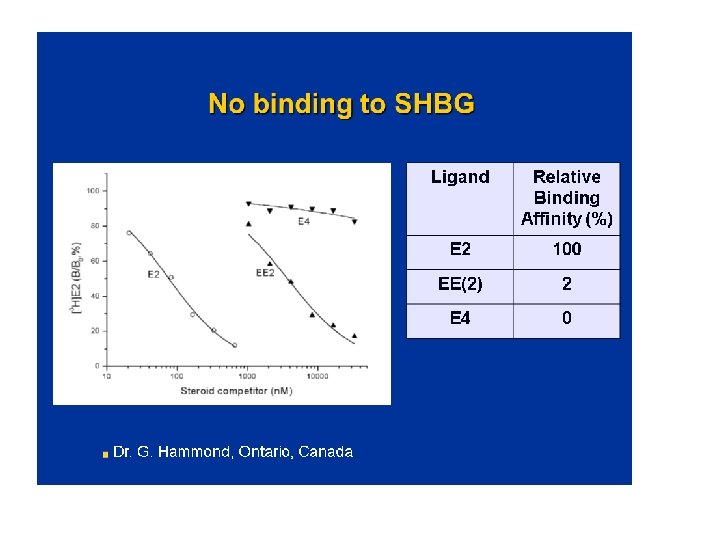
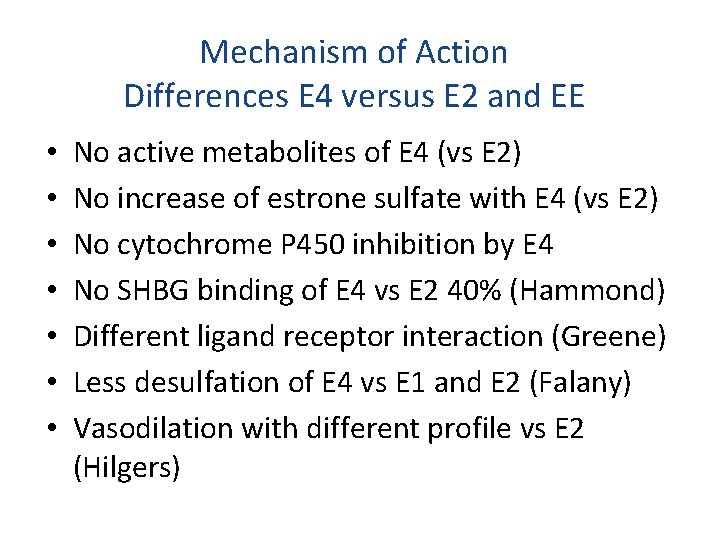
Mechanism of Action Differences E 4 versus E 2 and EE • • No active metabolites of E 4 (vs E 2) No increase of estrone sulfate with E 4 (vs E 2) No cytochrome P 450 inhibition by E 4 No SHBG binding of E 4 vs E 2 40% (Hammond) Different ligand receptor interaction (Greene) Less desulfation of E 4 vs E 1 and E 2 (Falany) Vasodilation with different profile vs E 2 (Hilgers)

Estetrol vs EE Expected advantages • Less subjective side effects (breast tension and tenderness, weight gain, edema, nausea, abdominal bloating, headache, mood changes) • Less interference with liver function • Improved cardiovascular safety, especially less Venous Thrombo-Embolism (VTE) • Less gall bladder disease • E 4 is an anti-estrogen for the breast

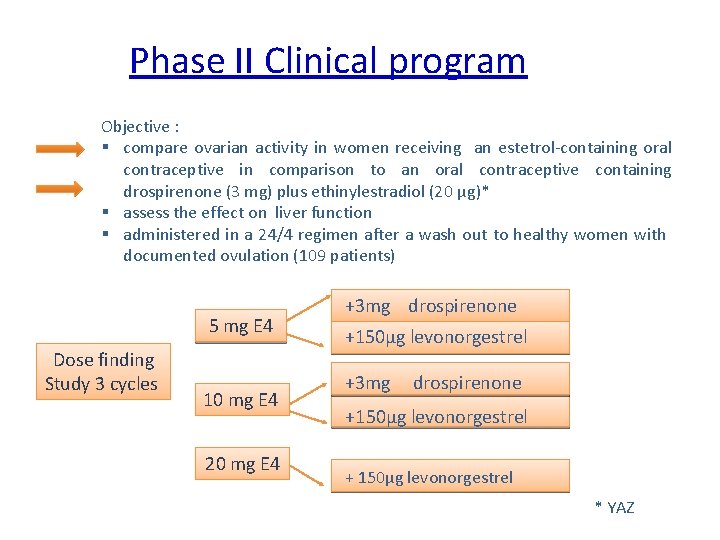
Phase II Clinical program Objective : § compare ovarian activity in women receiving an estetrol-containing oral contraceptive in comparison to an oral contraceptive containing drospirenone (3 mg) plus ethinylestradiol (20 µg)* § assess the effect on liver function § administered in a 24/4 regimen after a wash out to healthy women with documented ovulation (109 patients) 5 mg E 4 Dose finding Study 3 cycles 10 mg E 4 20 mg E 4 +3 mg drospirenone +150µg levonorgestrel +3 mg drospirenone +150µg levonorgestrel + 150µg levonorgestrel * YAZ

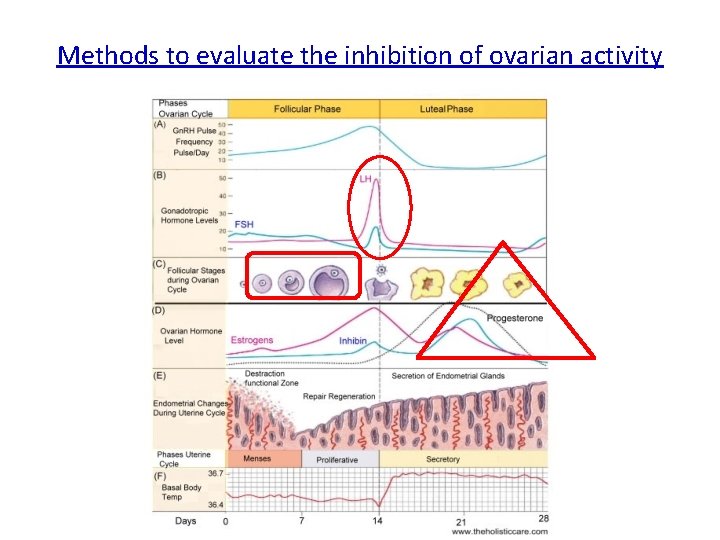
Methods to evaluate the inhibition of ovarian activity

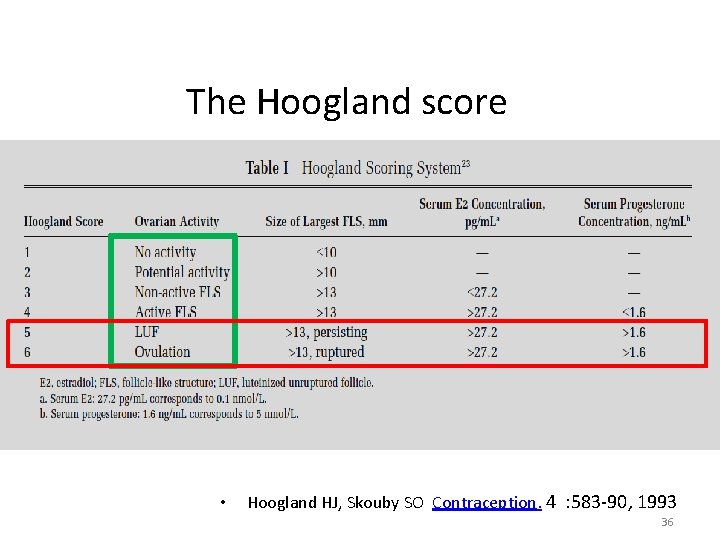
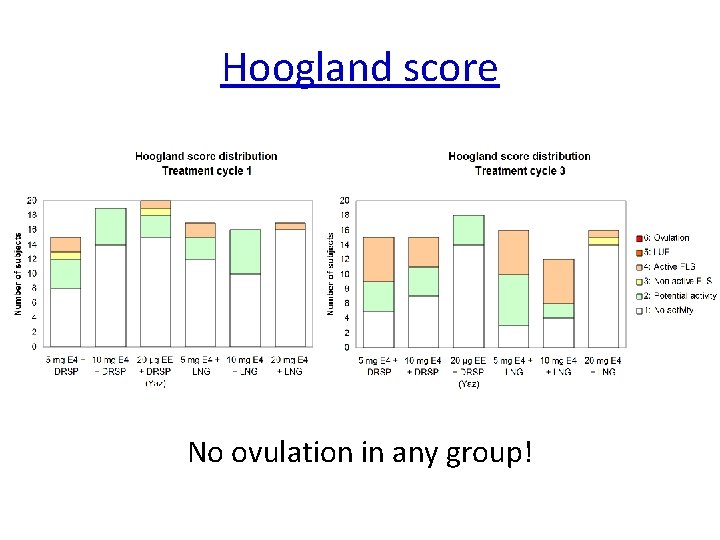
The Hoogland score • 24 November 2020 Hoogland HJ, Skouby SO Contraception. 4 : 583 -90, 1993 36

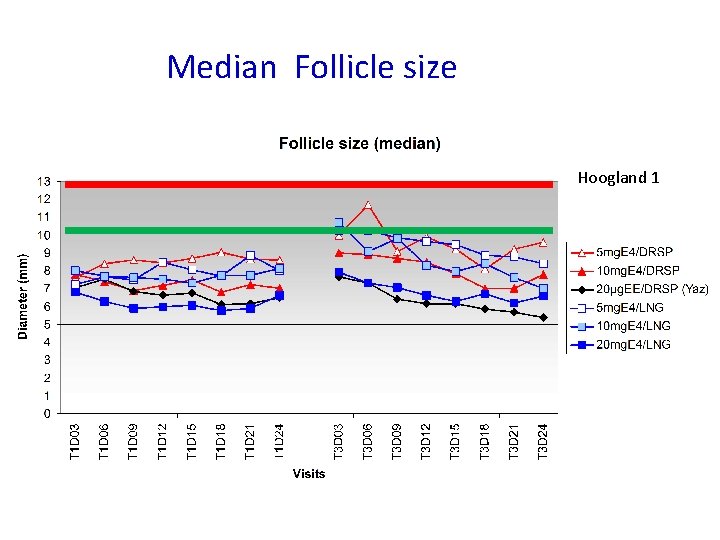
Median Follicle size Hoogland 1

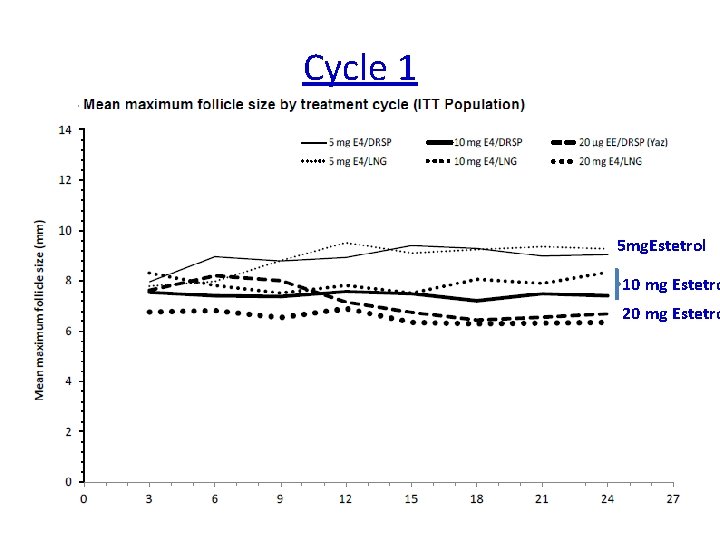
Cycle 1 5 mg. Estetrol 10 mg Estetro 20 mg Estetro

Cycle 3 <13 mm 5 mg Estetrol ≤ 10 mm 10 mg Estetrol 20 mg Estetrol

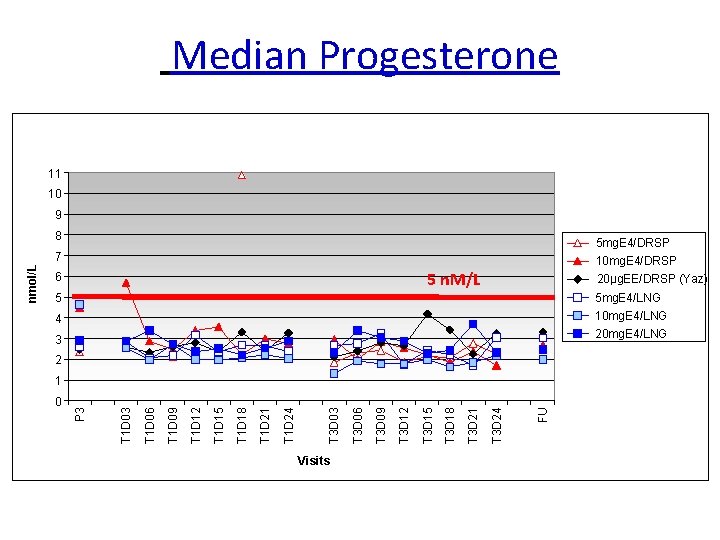
Median Progesterone 11 10 9 8 5 mg. E 4/DRSP 10 mg. E 4/DRSP 5 n. M/L 6 20μg. EE/DRSP (Yaz) 5 5 mg. E 4/LNG 4 10 mg. E 4/LNG 20 mg. E 4/LNG 3 2 Visits FU T 3 D 24 T 3 D 21 T 3 D 18 T 3 D 15 T 3 D 12 T 3 D 09 T 3 D 06 T 3 D 03 T 1 D 24 T 1 D 21 T 1 D 18 T 1 D 15 T 1 D 12 T 1 D 09 T 1 D 06 0 T 1 D 03 1 P 3 nmol/L 7

Hoogland score No ovulation in any group!

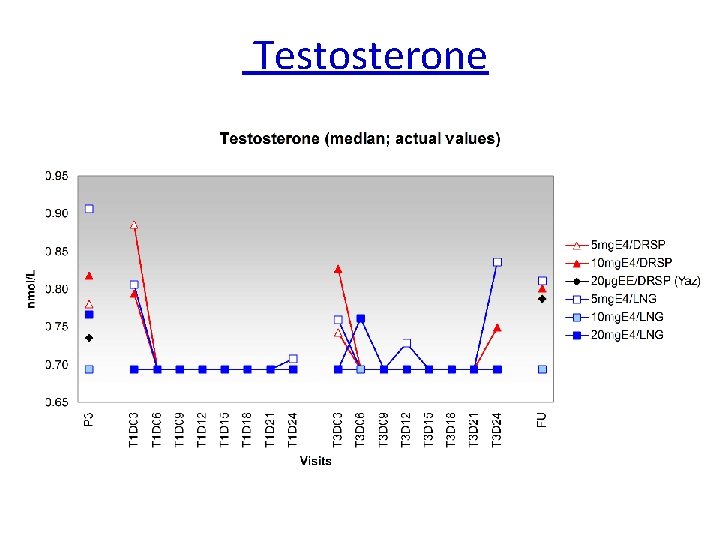
Testosterone

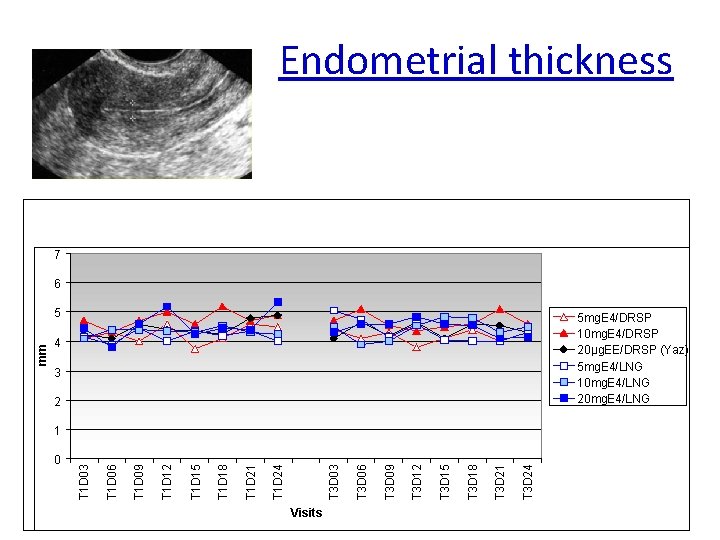
Endometrial thickness 7 6 5 mg. E 4/DRSP 10 mg. E 4/DRSP 20μg. EE/DRSP (Yaz) 5 mg. E 4/LNG 10 mg. E 4/LNG 20 mg. E 4/LNG 4 3 2 Visits T 3 D 24 T 3 D 21 T 3 D 18 T 3 D 15 T 3 D 12 T 3 D 09 T 3 D 06 T 3 D 03 T 1 D 24 T 1 D 21 T 1 D 18 T 1 D 15 T 1 D 12 T 1 D 09 0 T 1 D 06 1 T 1 D 03 mm 5

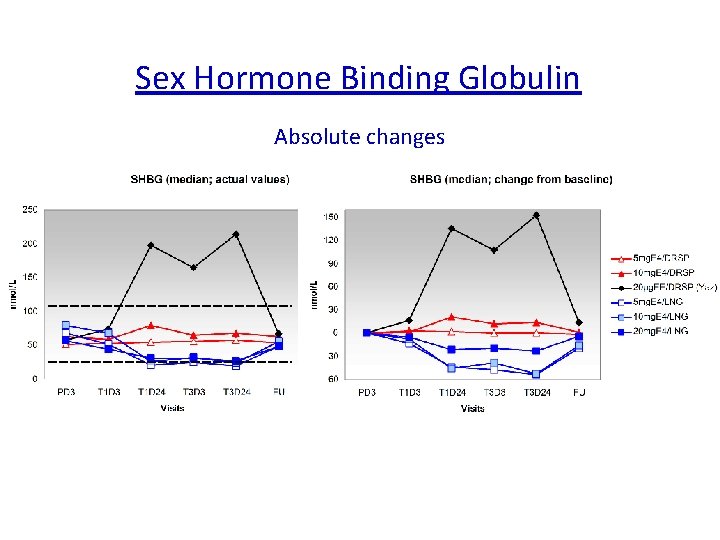
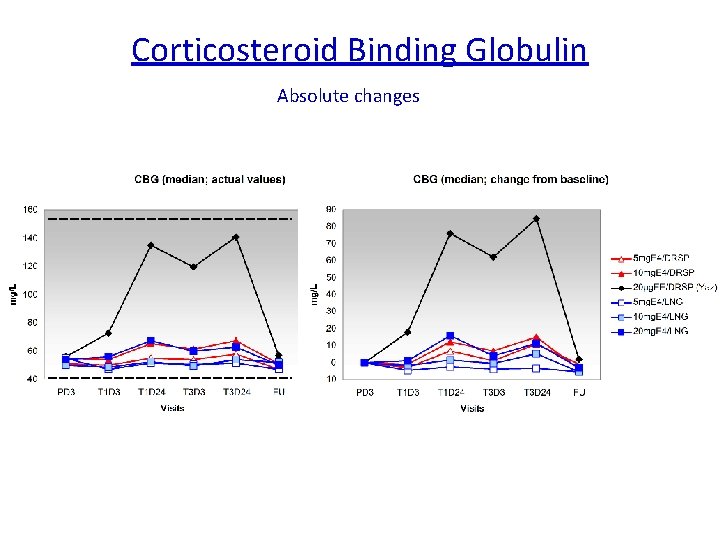
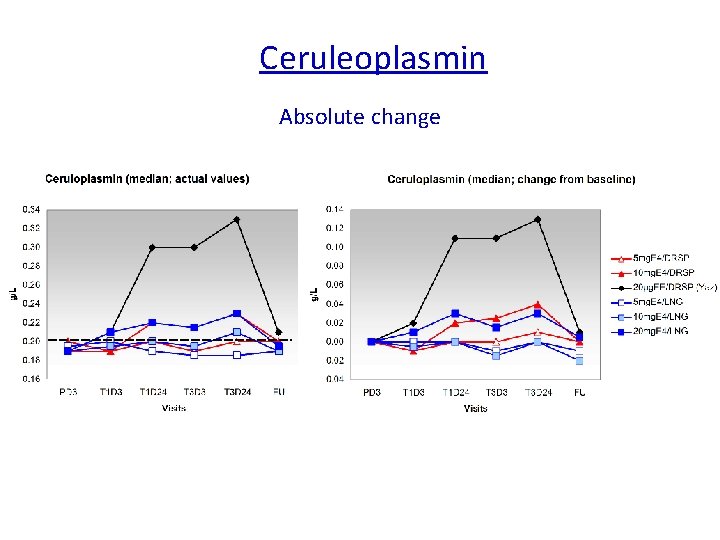
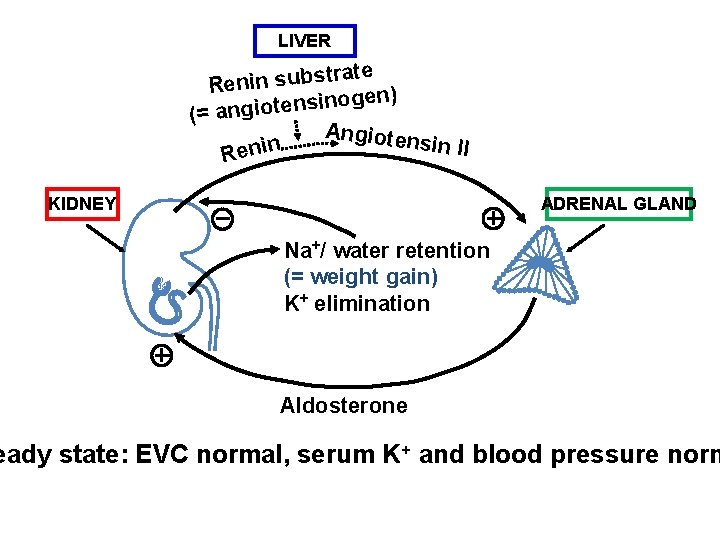
Is Estetrol, a « liver friendly » estrogen ? The synthesis of several plasma proteins is at least in part controlled by estrogens, particularly , when given by the oral route : o some coagulation factors o SHBG, o CBG, o Ceruleoplasmin, o Angiotensinogen…. High androgen levels decrease SHBG plasma levels, whereas high GH, or estrogen increase them. It is considered therefore as a marker of estrogenic imprinting on liver.

Sex Hormone Binding Globulin Absolute changes Reference range SHBG: 26 – 110 nmol/L

Corticosteroid Binding Globulin Absolute changes Reference range CBG: 40 – 154 mg/L

Ceruleoplasmin Absolute change Reference range Ceruloplasmin: 0. 2 – 0. 6 g/L

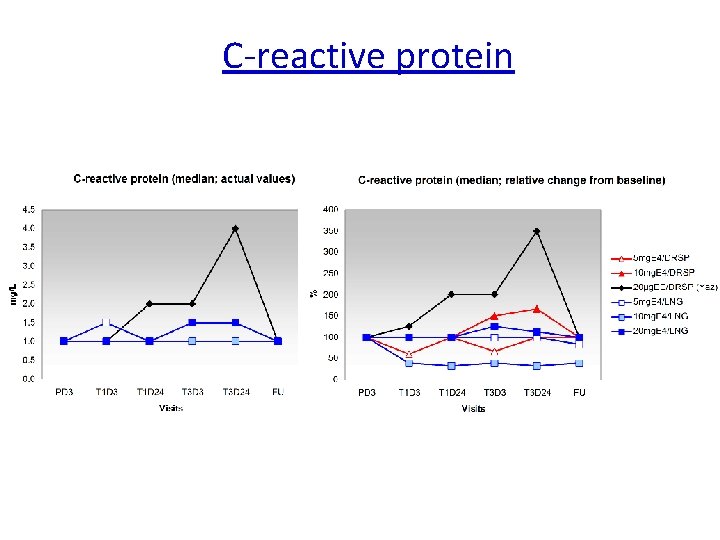
C-reactive protein Relative change Reference range CRP: < 5 mg/L

LIVER trate s b u s n i n e R en) g o n i s n e t o i (= ang Angioten sin II n Reni KIDNEY ADRENAL GLAND Na+/ water retention (= weight gain) K+ elimination Aldosterone eady state: EVC normal, serum K+ and blood pressure norm

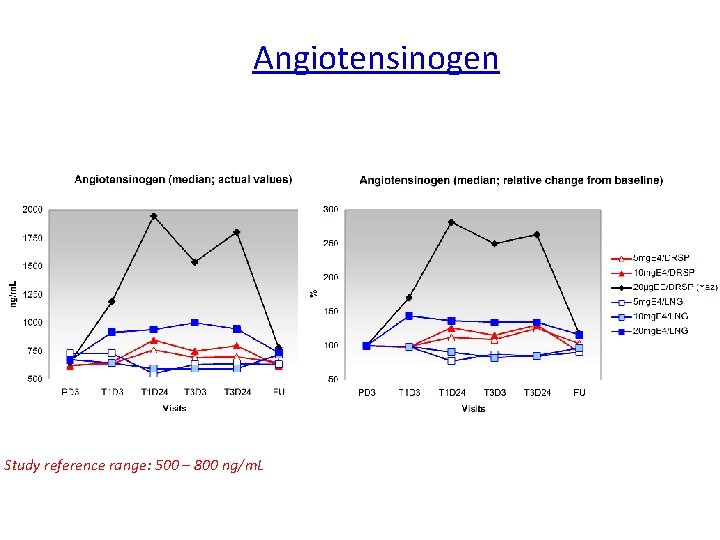
Angiotensinogen Relative change Reference range Angiotensinogen: not available Study reference range: 500 – 800 ng/m. L

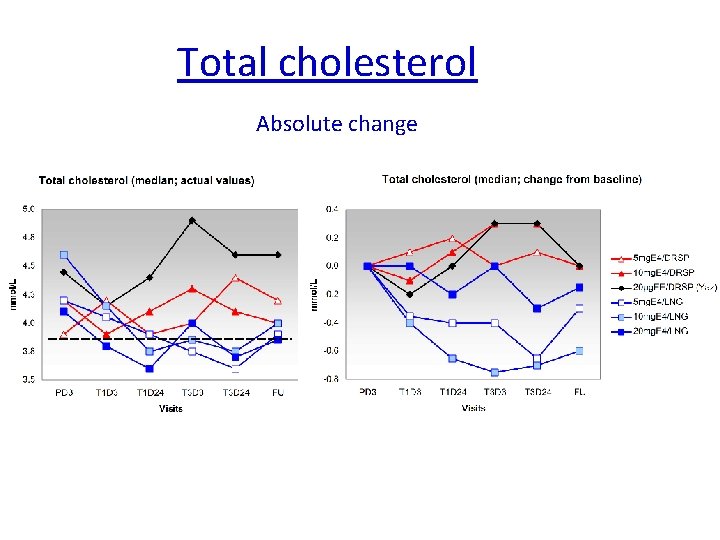
Total cholesterol Absolute change Reference range Total cholesterol: 3. 9 – 6. 5 mmol/L

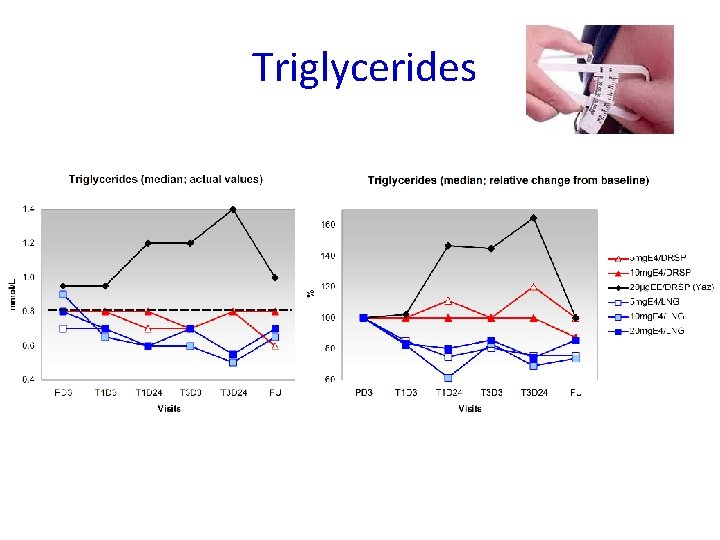
Triglycerides Reference range Triglycerides: 0. 8 – 2. 0 mmol/L

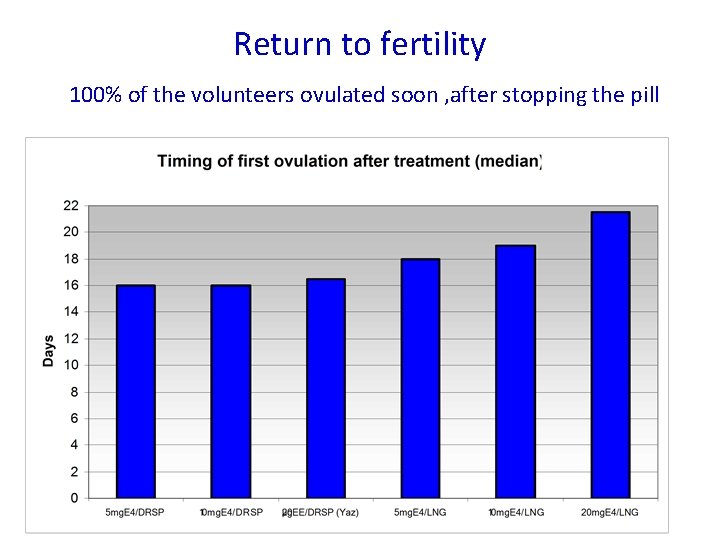
Return to fertility 100% of the volunteers ovulated soon , after stopping the pill

Outstanding rapid return to fertility : Reversibilty is excellent!

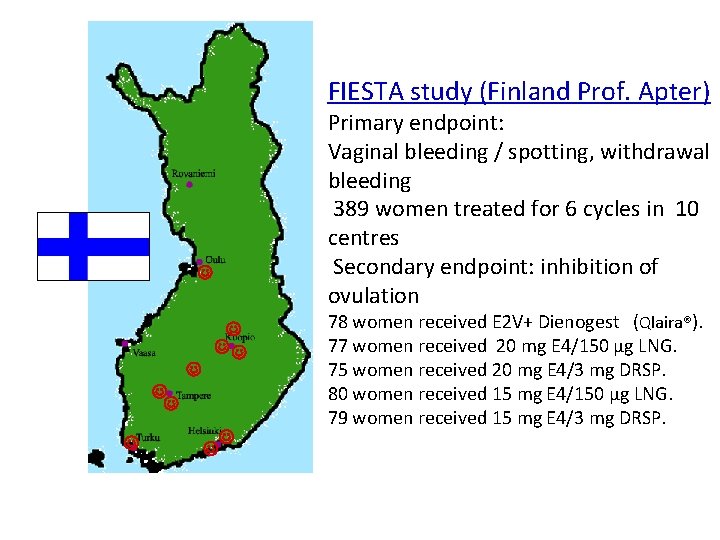
FIESTA study (Finland Prof. Apter) 27 August 2010 Primary endpoint: Vaginal bleeding / spotting, withdrawal bleeding 389 women treated for 6 cycles in 10 FIESTA study Finland centres *Primary endpoint: Secondary endpoint: inhibition of ovulation Vaginal bleeding 78 women received E 2 V+ Dienogest (Qlaira®). 77 women received 20 mg E 4/150 μg LNG. 75 women received 20 mg E 4/3 mg DRSP. 80 women received 15 mg E 4/150 μg LNG. 79 women received 15 mg E 4/3 mg DRSP. * 400 women/6 cycles * 10 centres * Clinically completed September 2011 FIESTA (ES-C 02) 55

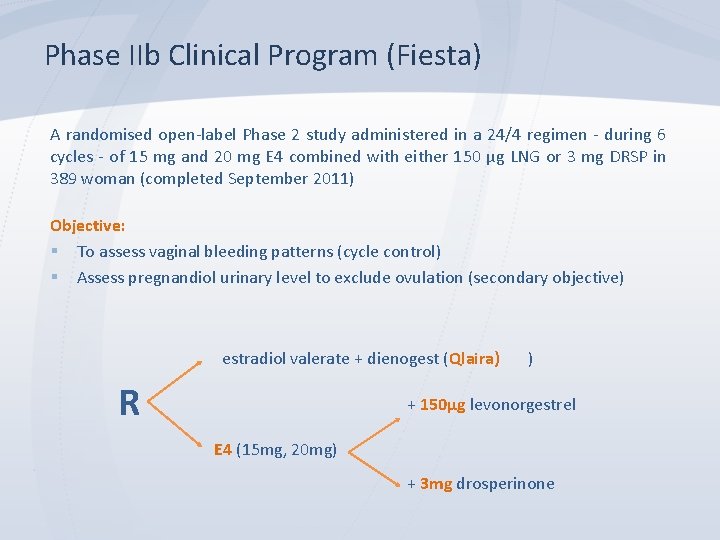
Phase IIb Clinical Program (Fiesta) A randomised open-label Phase 2 study administered in a 24/4 regimen - during 6 cycles - of 15 mg and 20 mg E 4 combined with either 150 μg LNG or 3 mg DRSP in 389 woman (completed September 2011) Objective: § To assess vaginal bleeding patterns (cycle control) § Assess pregnandiol urinary level to exclude ovulation (secondary objective) estradiol valerate + dienogest (Qlaira) ) R + 150µg levonorgestrel E 4 (15 mg, 20 mg) + 3 mg drosperinone

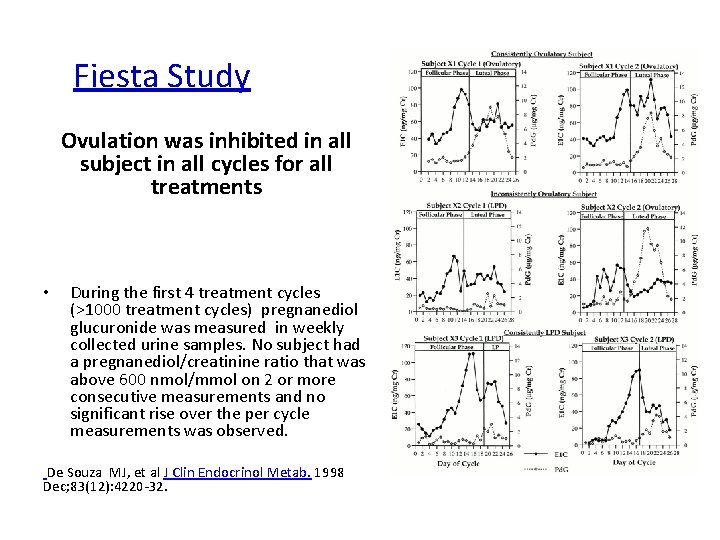
Fiesta Study Ovulation was inhibited in all subject in all cycles for all treatments • During the first 4 treatment cycles (>1000 treatment cycles) pregnanediol glucuronide was measured in weekly collected urine samples. No subject had a pregnanediol/creatinine ratio that was above 600 nmol/mmol on 2 or more consecutive measurements and no significant rise over the per cycle measurements was observed. De Souza MJ, et al J Clin Endocrinol Metab. 1998 Dec; 83(12): 4220 -32.

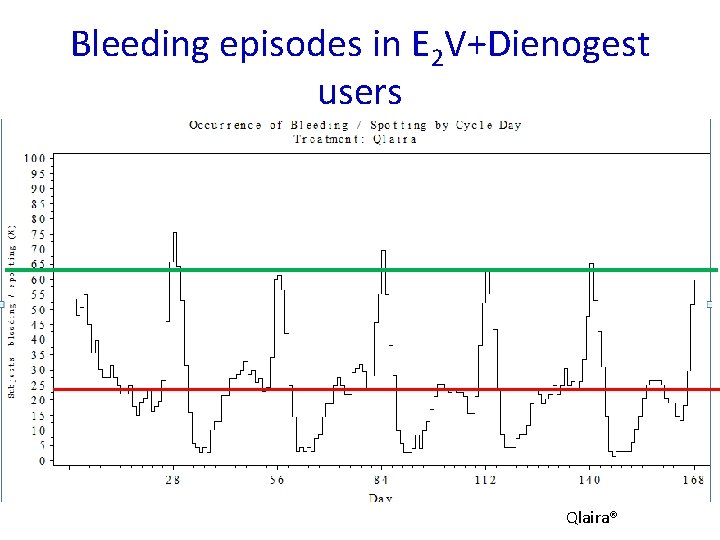
Bleeding episodes in E 2 V+Dienogest users Qlaira®

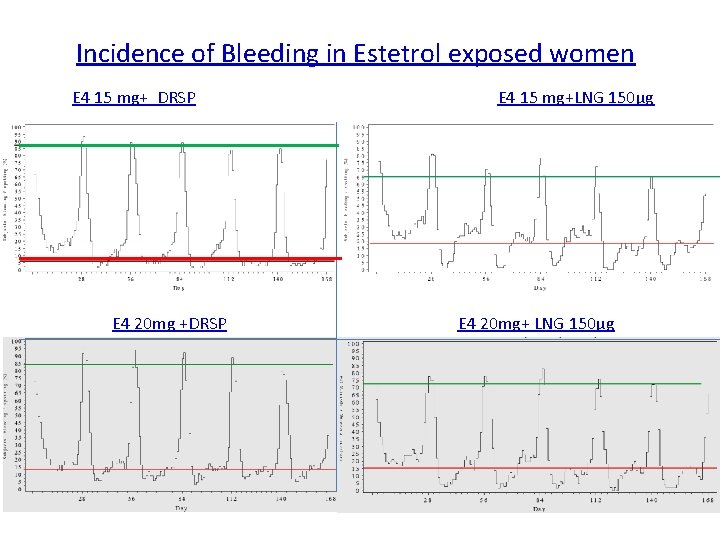
Incidence of Bleeding in Estetrol exposed women E 4 15 mg+ DRSP E 4 20 mg +DRSP E 4 15 mg+LNG 150µg E 4 20 mg+ LNG 150µg

Conclusions Fiesta study § Excellent bleeding profile § < 10% women experience a spotting, bleeding episode § > 90% experience a withdrawal bleeding § No ovulation in over 1, 500 cycles § Considerably better bleeding profile than the E 2 containing pill

Potency E 4 vs E 2 • Receptor affinity of E 4 is about 5% compared to E 2 which has created the perception of a weak estrogen but: 1) Receptor affinity of tamoxiphene, SERM’s and tibolone-metabolites is also about 5% compared to E 2 2) Bioavailability of E 4 is at least 80% vs 5% for E 2 3) No SHBG binding/inactivation of E 4 vs E 2 (40%) 4) Elimination half life of E 4 is 28 hrs vs E 2 1 -2 hrs and micronised E 2 10 -12 hrs • Estetrol is very safe in tox studies and can be dosed much higher than E 2 without side effects