Esterification and dehydration of alcohols Keywords Esterification elimination




- Slides: 13

Esterification and dehydration of alcohols Keywords: Esterification, elimination, ester, dehydration Starter: Use molecular models to make various alcohol molecules

Learning Objectives A B C ü Model the esterification of alcohols with carboxylic acids ü Describe the elimination of H 2 O from alcohols to form alkenes. ü Name the esters formed by esterification reactions

Esterification – the reaction of an alcohol with a carboxylic acid to produce an ester and water Elimination – breaking up 1 molecule into 2 molecules Dehydration – an elimination reaction in which water is removed from a saturated molecule to make an unsaturated molecule

Esterification An ester is formed when an alcohol is warmed with a carboxylic acid in the presence of an acid catalyst The reaction is known as esterification Concentrated sulfuric acid is often used as the acid catalyst

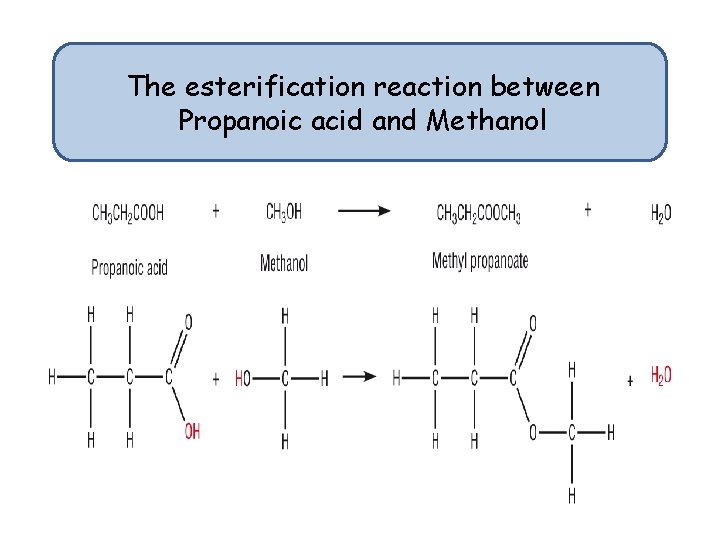
The esterification reaction between Propanoic acid and Methanol

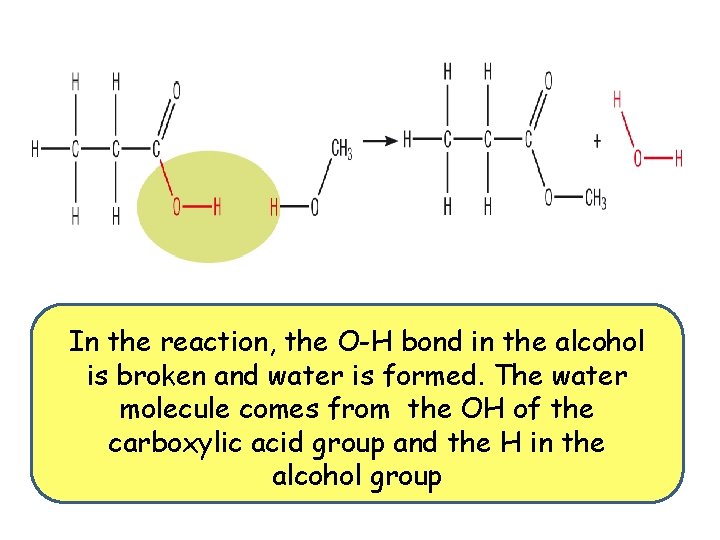
In the reaction, the O-H bond in the alcohol is broken and water is formed. The water molecule comes from the OH of the carboxylic acid group and the H in the alcohol group

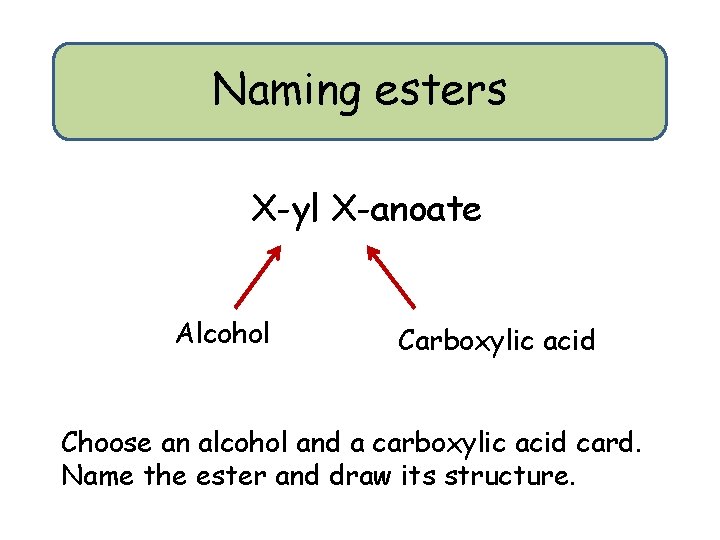
Naming esters X-yl X-anoate Alcohol Carboxylic acid Choose an alcohol and a carboxylic acid card. Name the ester and draw its structure.

Esters are used as adhesives and solvents in the chemical industry. The flavours and fragrances of different esters are widely used to produce food flavourings and perfumes.

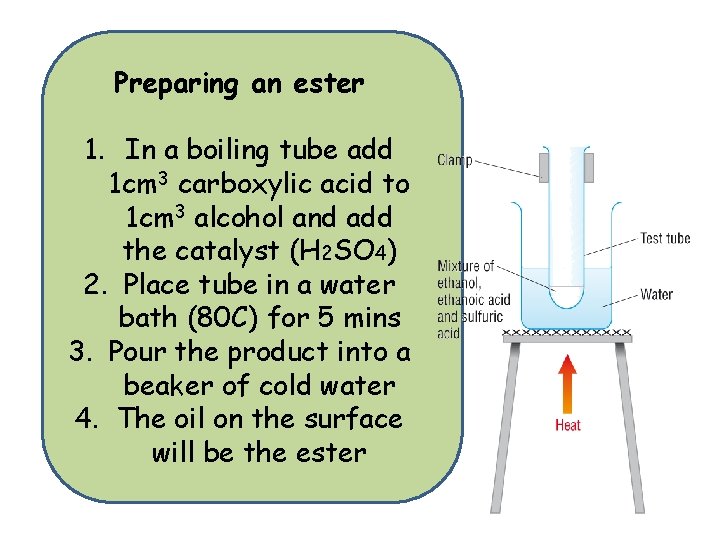
Preparing an ester 1. In a boiling tube add 1 cm 3 carboxylic acid to 1 cm 3 alcohol and add the catalyst (H 2 SO 4) 2. Place tube in a water bath (80 C) for 5 mins 3. Pour the product into a beaker of cold water 4. The oil on the surface will be the ester

Dehydration of an alcohol An alcohol can be dehydrated to form an alkene in the presence of an acid catalyst (Conc phosphoric acid or conc sulphuric acid) This is an elimination reaction Alcohol is heated under reflux with the catalyst for 40 minutes.

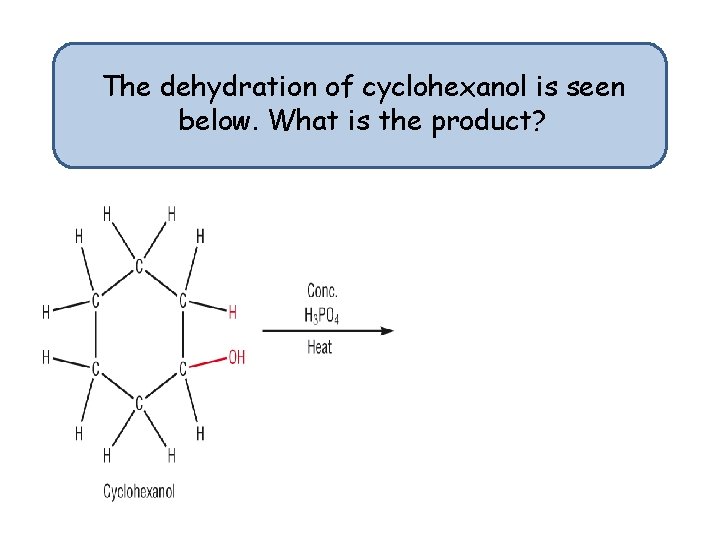
The dehydration of cyclohexanol is seen below. What is the product?

Plenary Pg 155 Questions 1, 2 and 3

Methanol Methanoic acid Ethanol Ethanoic acid Propanol Propanoic acid Butanol Butanoic acid Pentanol Pentanoic acid Hexanol Hexanoic acid