Establishment of a Comparability Strategy to Support a

Establishment of a Comparability Strategy to Support a Cell Line Change During Clinical Development of a Monoclonal Antibody Bryan J. Harmon www. diahome. org

Disclaimer The views and opinions expressed in the following Power. Point slides are those of the individual presenter and should not be attributed to Drug Information Association, Inc. (“DIA”), its directors, officers, employees, volunteers, members, chapters, councils, Special Interest Area Communities or affiliates, or any organization with which the presenter is employed or affiliated. These Power. Point slides are the intellectual property of the individual presenter and are protected under the copyright laws of the United States of America and other countries. Used by permission. All rights reserved. Drug Information Association, DIA and DIA logo are registered trademarks or trademarks of Drug Information Association Inc. All other trademarks are the property of their respective owners. www. diahome. org

Outline • Drivers for Cell Line Changes • Elements of Comparability Strategy • Case Studies • Conclusions www. diahome. org

Cell Line Changes During Clinical Development Driver Quality risk with initial cell line Examples • Genetic splicing or mutation identified • ASM exposure during cell line generation • Lack of assurance of clonality • Insufficient titer Initial cell line is • Insufficient cell line stability not commercially • Not consistent with manufacturing viable platform • Intellectual property issues www. diahome. org
Types of Cell Line Changes • Additional round of cloning • Different clone from same host cell line • Different host cell line Cell line changes: • Are considered the biggest risk among process changes • Have been practiced very conservatively in the industry www. diahome. org
Elements of Integrated Comparability Strategy 1. Host cell line & clone selection criteria 2. Analytical comparability testing strategy 3. In vitro biological testing 4. Nonclinical PK, PD & immunogenicity assessments 5. Clinical assessments www. diahome. org Need for & extent of each element driven by risk assessments

Risk Assessments – FMEA Analysis 1. Severity – impact on toxicity, safety, efficacy or PK/PD 2. Occurrence – likelihood of being outside preclinical & clinical experience (process capability, control & robustness) 3. Detection – capability of analytical methods to detect occurrence Risk Rating = Severity x Occurrence x Detection Risk assessments must be cross-functional (toxicology, medical, analytical, process scientists) www. diahome. org

Host Cell Line & Clone Selection Criteria In evaluating risk of cell line change, must consider: • Post-translational modification capabilities of potential new host cell line • Clonal variability of chosen host cell line in product quality attributes • Capability to mitigate comparability risks through process development/optimization www. diahome. org
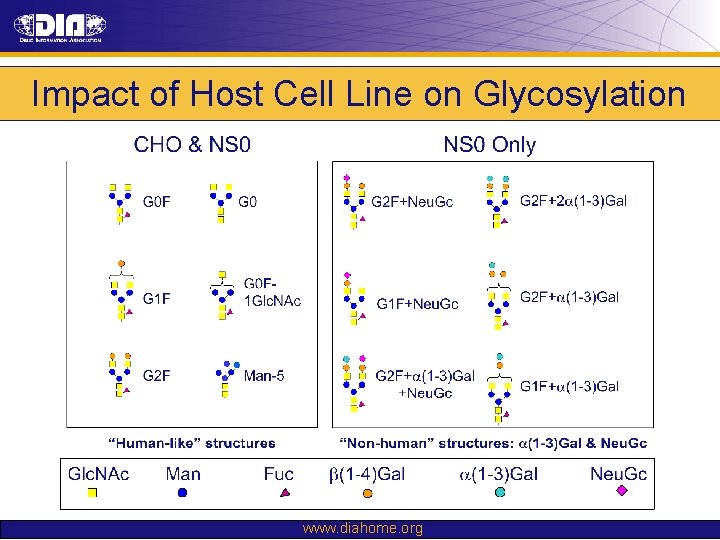
Impact of Host Cell Line on Glycosylation www. diahome. org

Impact of Host Cell Line on Glycosylation CE-LIF Oligosaccharide Profiling www. diahome. org
Risks of Cell Line Changes • Different host cell line • Different clone from same host cell line • Additional round of cloning www. diahome. org Increasing risk to CQAs of molecule
Host Cell Line & Clone Selection Criteria In evaluating risk of cell line change, must consider: • Post-translational modification capabilities of potential new host cell line • Clonal variability of chosen host cell line in product quality attributes • Capability to mitigate comparability risks through process development/optimization www. diahome. org
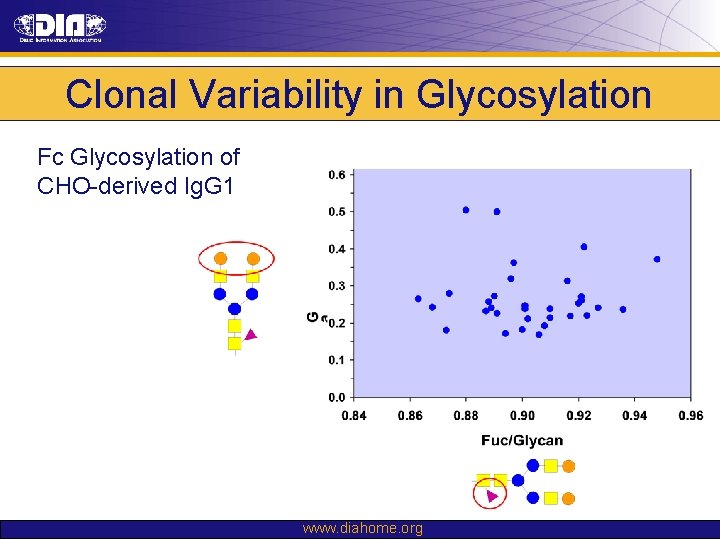
Clonal Variability in Glycosylation Fc Glycosylation of CHO-derived Ig. G 1 www. diahome. org
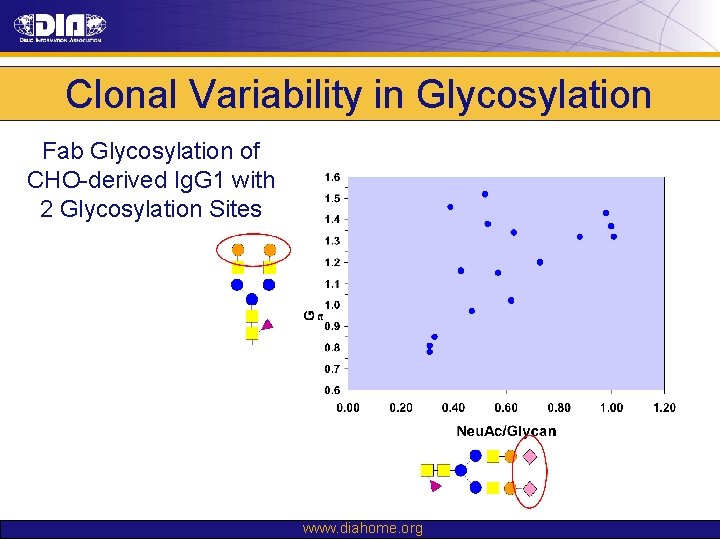
Clonal Variability in Glycosylation Fab Glycosylation of CHO-derived Ig. G 1 with 2 Glycosylation Sites www. diahome. org
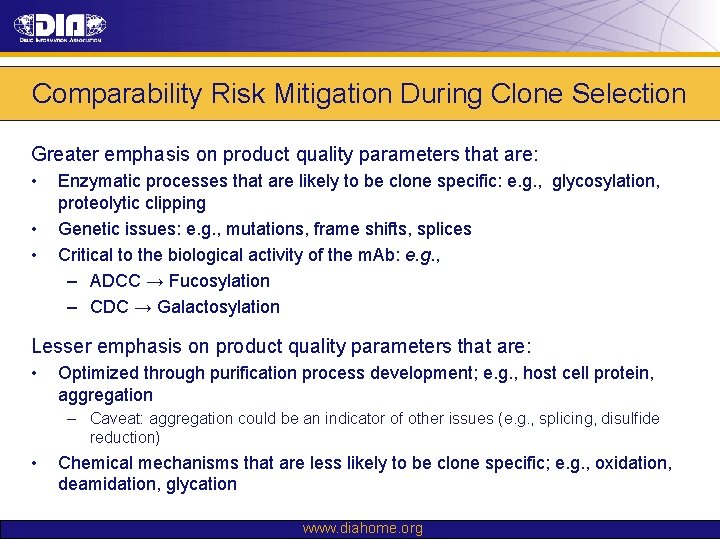
Comparability Risk Mitigation During Clone Selection Greater emphasis on product quality parameters that are: • • • Enzymatic processes that are likely to be clone specific: e. g. , glycosylation, proteolytic clipping Genetic issues: e. g. , mutations, frame shifts, splices Critical to the biological activity of the m. Ab: e. g. , – ADCC → Fucosylation – CDC → Galactosylation Lesser emphasis on product quality parameters that are: • Optimized through purification process development; e. g. , host cell protein, aggregation – Caveat: aggregation could be an indicator of other issues (e. g. , splicing, disulfide reduction) • Chemical mechanisms that are less likely to be clone specific; e. g. , oxidation, deamidation, glycation www. diahome. org
Host Cell Line & Clone Selection Criteria In evaluating risk of cell line change, must consider: • Post-translational modification capabilities of potential new host cell line • Clonal variability of chosen host cell line in product quality attributes • Capability to mitigate comparability risks through process development/optimization www. diahome. org
Physico-Chemical Comparability Testing Assess impact of cell line change on CQAs of MAb based upon risk assessment of quality attributes • Additional testing to satisfy regulatory concerns; e. g. , – • Glycosylation analysis for MAb whose MOA is not dependent upon effector function Co-mixture analysis of representative lots where appropriate (e. g. , LC-MS peptide mapping, SEC, CEX, CE-SDS) Assessment of impact on degradation mechanisms (e. g. , stressed or accelerated stability study) Pre-defined acceptance criteria: • • – At early stages of development: • • – Insufficient data to establish statistical limits tighter than specifications at early stages of development Qualitative criteria for characterization assays Allowance for investigative testing (e. g. , source of differences in charge heterogeneity) www. diahome. org
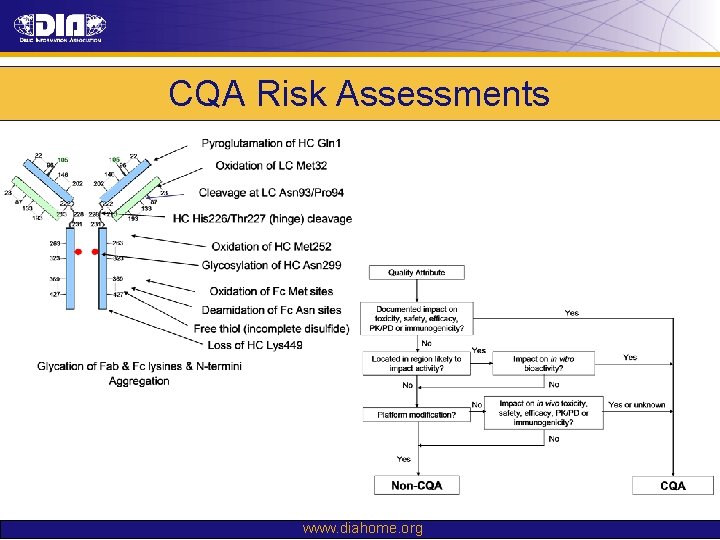
CQA Risk Assessments www. diahome. org
Physico-Chemical Comparability Testing Assess impact of cell line change on CQAs of MAb based upon risk assessment of quality attributes • Additional testing to satisfy regulatory concerns; e. g. , – • Glycosylation analysis for MAb whose MOA is not dependent upon effector function Co-mixture analysis of representative lots where appropriate (e. g. , LC-MS peptide mapping, SEC, CEX, CE-SDS) Assessment of impact on degradation mechanisms (e. g. , stressed or accelerated stability study) Pre-defined acceptance criteria: • • – At early stages of development: • • – Insufficient data to establish statistical limits tighter than specifications at early stages of development Qualitative criteria for characterization assays Allowance for investigative testing (e. g. , source of differences in charge heterogeneity) www. diahome. org
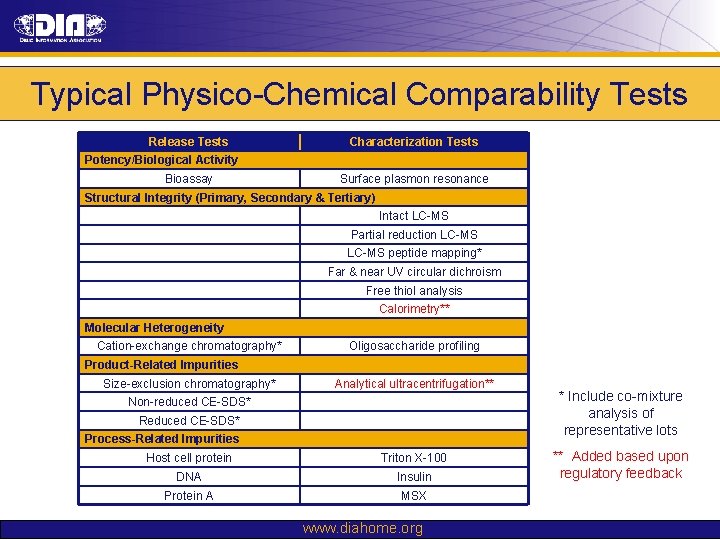
Typical Physico-Chemical Comparability Tests Release Tests Characterization Tests Potency/Biological Activity Bioassay Surface plasmon resonance Structural Integrity (Primary, Secondary & Tertiary) Intact LC-MS Partial reduction LC-MS peptide mapping* Far & near UV circular dichroism Free thiol analysis Calorimetry** Molecular Heterogeneity Cation-exchange chromatography* Oligosaccharide profiling Product-Related Impurities Size-exclusion chromatography* Analytical ultracentrifugation** Non-reduced CE-SDS* Reduced CE-SDS* Process-Related Impurities Host cell protein Triton X-100 DNA Insulin Protein A MSX www. diahome. org * Include co-mixture analysis of representative lots ** Added based upon regulatory feedback
Physico-Chemical Comparability Testing Assess impact of cell line change on CQAs of MAb based upon risk assessment of quality attributes • Additional testing to satisfy regulatory concerns; e. g. , – • Glycosylation analysis for MAb whose MOA is not dependent upon effector function Co-mixture analysis of representative lots where appropriate (e. g. , LC-MS peptide mapping, SEC, CEX, CE-SDS) Assessment of impact on degradation mechanisms (e. g. , stressed or accelerated stability study) Pre-defined acceptance criteria: • • – At early stages of development: • • – Insufficient data to establish statistical limits tighter than specifications at early stages of development Qualitative criteria for characterization assays Allowance for investigative testing (e. g. , source of differences in charge heterogeneity) www. diahome. org
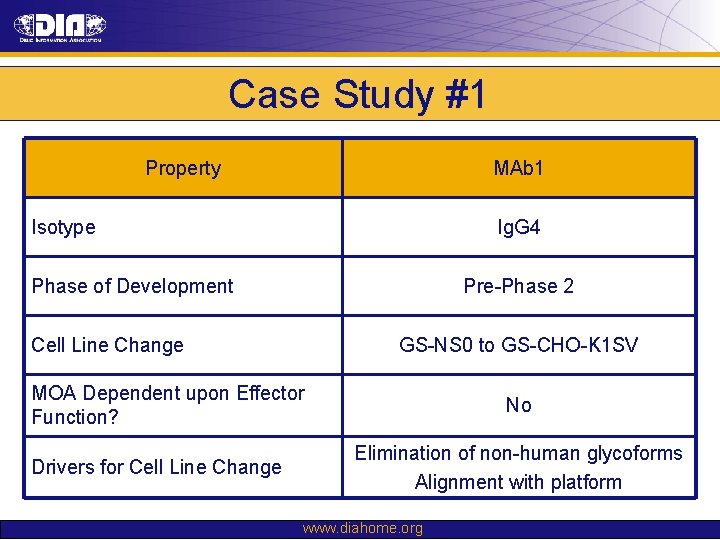
Case Study #1 Property MAb 1 Isotype Ig. G 4 Phase of Development Pre-Phase 2 Cell Line Change GS-NS 0 to GS-CHO-K 1 SV MOA Dependent upon Effector Function? Drivers for Cell Line Change No Elimination of non-human glycoforms Alignment with platform www. diahome. org

Case Study #1 Prior Knowledge: • Experience in GS-NS 0 to GS-CHO-K 1 SV cell line changes suggested risk of: – Changes in glycosylation profile – Changes in charge heterogeneity resulting from differences in proportions of charge variants Risk Assessment: • Expected differences presented low risk to the safety and efficacy of molecule Comparability Strategy: • Extraordinary efforts would not be made in clone selection and process development to eliminate these differences • Demonstrate comparability through: – Physico-chemical testing – In vitro biological assays – Non-clinical in vivo PK, PD and immunogenicity studies www. diahome. org
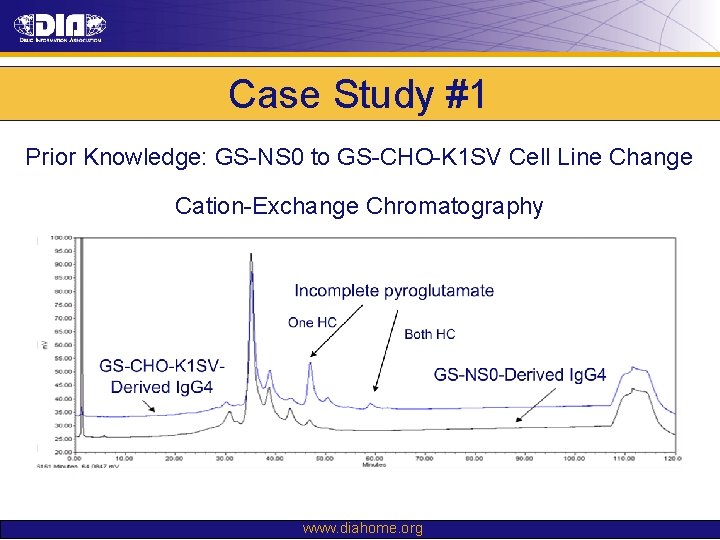
Case Study #1 Prior Knowledge: GS-NS 0 to GS-CHO-K 1 SV Cell Line Change Cation-Exchange Chromatography www. diahome. org

Case Study #1 Prior Knowledge: • Experience in GS-NS 0 to GS-CHO-K 1 SV cell line changes suggested risk of: – Changes in glycosylation profile – Changes in charge heterogeneity resulting from differences in proportions of charge variants Risk Assessment: • Expected differences presented low risk to the safety and efficacy of molecule Comparability Strategy: • Extraordinary efforts would not be made in clone selection and process development to eliminate these differences • Demonstrate comparability through: – Physico-chemical testing – In vitro biological assays – Non-clinical in vivo PK, PD and immunogenicity studies www. diahome. org
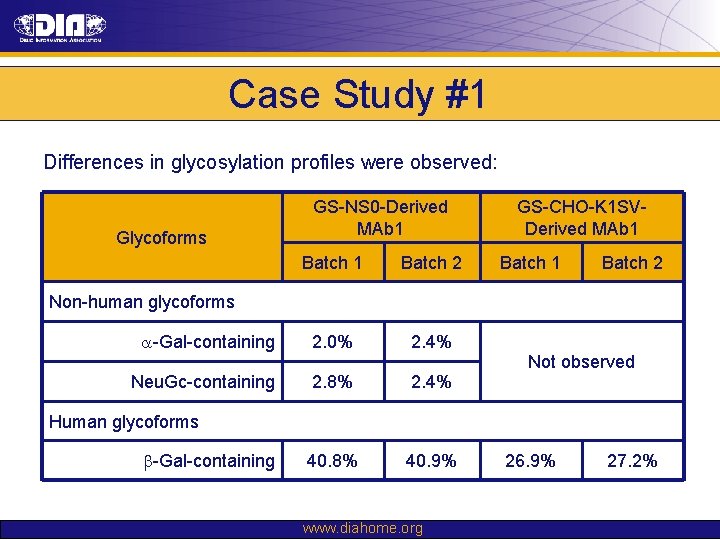
Case Study #1 Differences in glycosylation profiles were observed: Glycoforms GS-NS 0 -Derived MAb 1 Batch 2 a-Gal-containing 2. 0% 2. 4% Neu. Gc-containing 2. 8% 2. 4% 40. 8% 40. 9% GS-CHO-K 1 SVDerived MAb 1 Batch 2 Non-human glycoforms Not observed Human glycoforms b-Gal-containing www. diahome. org 26. 9% 27. 2%
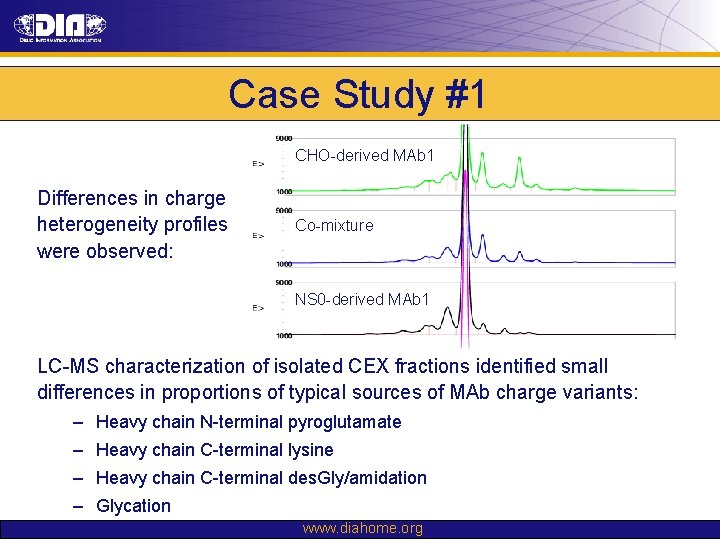
Case Study #1 CHO-derived MAb 1 Differences in charge heterogeneity profiles were observed: Co-mixture NS 0 -derived MAb 1 LC-MS characterization of isolated CEX fractions identified small differences in proportions of typical sources of MAb charge variants: – Heavy chain N-terminal pyroglutamate – Heavy chain C-terminal lysine – Heavy chain C-terminal des. Gly/amidation – Glycation www. diahome. org
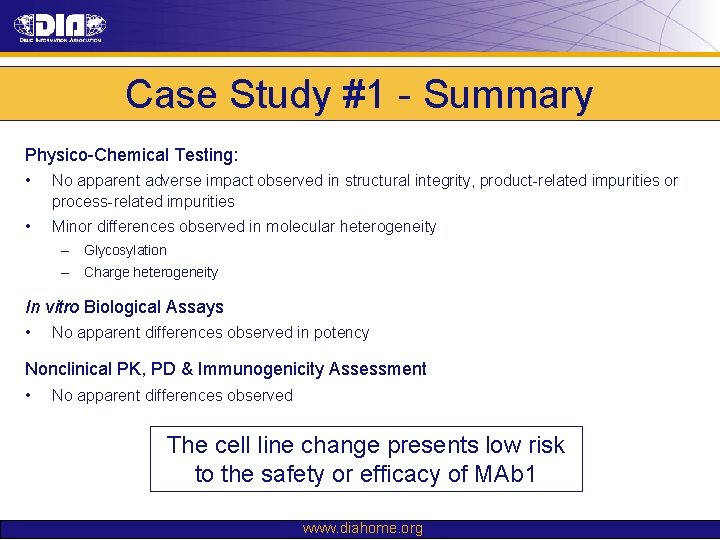
Case Study #1 - Summary Physico-Chemical Testing: • No apparent adverse impact observed in structural integrity, product-related impurities or process-related impurities • Minor differences observed in molecular heterogeneity – Glycosylation – Charge heterogeneity In vitro Biological Assays • No apparent differences observed in potency Nonclinical PK, PD & Immunogenicity Assessment • No apparent differences observed The cell line change presents low risk to the safety or efficacy of MAb 1 www. diahome. org
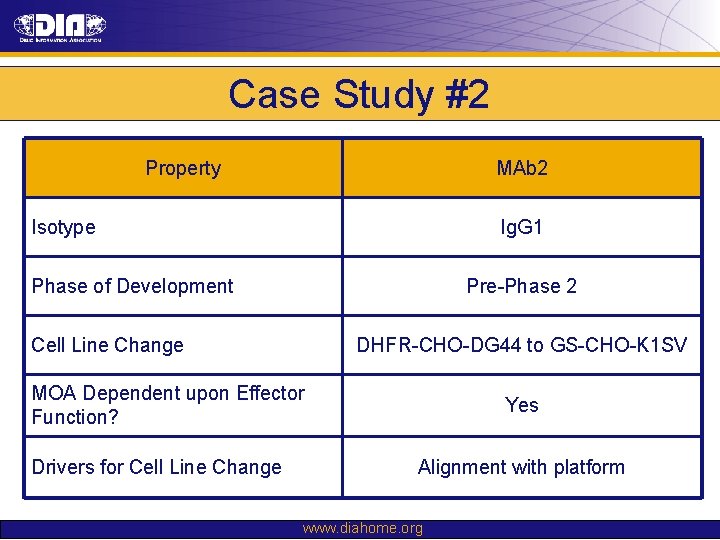
Case Study #2 Property MAb 2 Isotype Ig. G 1 Phase of Development Pre-Phase 2 Cell Line Change DHFR-CHO-DG 44 to GS-CHO-K 1 SV MOA Dependent upon Effector Function? Drivers for Cell Line Change Yes Alignment with platform www. diahome. org

Case Study #2 Prior Knowledge: • No experience in DHFR-CHO-DG 44 to GS-CHO-K 1 SV cell line changes • Knowledge of clonal variability suggested risk of changes in glycosylation profile: – Core fucosylation → impact ADCC activity – Terminal b-galactose → impact CDC activity Risk Assessment: • Changes in glycosylation could present significant risk to the safety and efficacy of molecule Comparability Strategy: • Glycosylation as criterion for clone selection to mitigate comparability risk – Fucosylation prioritized based upon proposed MOA • Demonstrate comparability through: – Physico-chemical testing – In vitro biological assays (including ADCC & CDC) – Non-clinical in vivo PK, PD & immunogenicity studies www. diahome. org
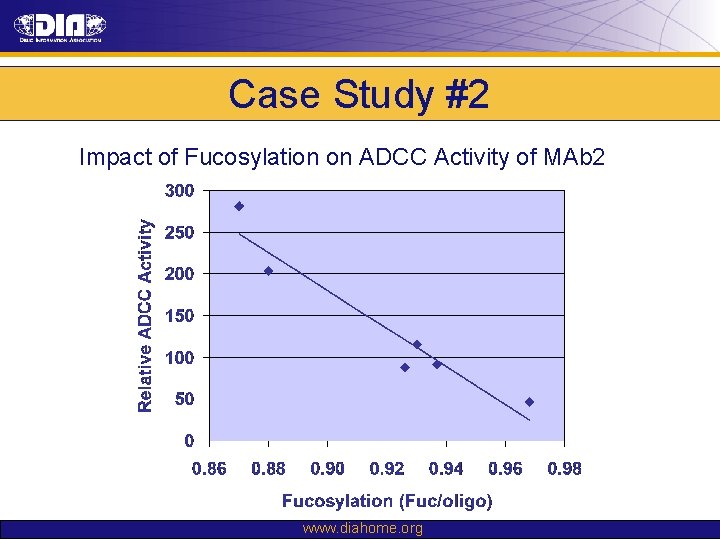
Case Study #2 Impact of Fucosylation on ADCC Activity of MAb 2 www. diahome. org

Case Study #2 Prior Knowledge: • No experience in DHFR-CHO-DG 44 to GS-CHO-K 1 SV cell line changes • Knowledge of clonal variability suggested risk of changes in glycosylation profile: – Core fucosylation → impact ADCC activity – Terminal b-galactose → impact CDC activity Risk Assessment: • Changes in glycosylation could present significant risk to the safety and efficacy of molecule Comparability Strategy: • Glycosylation as criterion for clone selection to mitigate comparability risk – Fucosylation prioritized based upon proposed MOA • Demonstrate comparability through: – Physico-chemical testing – In vitro biological assays (including ADCC & CDC) – Non-clinical in vivo PK, PD & immunogenicity studies www. diahome. org
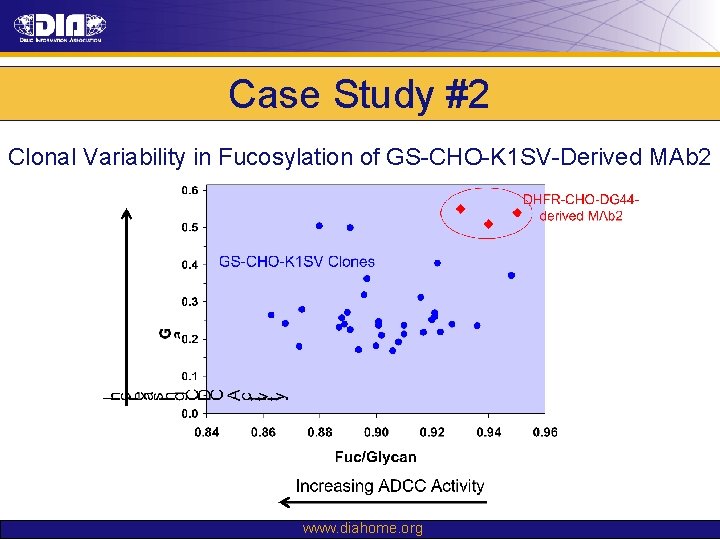
Case Study #2 Clonal Variability in Fucosylation of GS-CHO-K 1 SV-Derived MAb 2 www. diahome. org
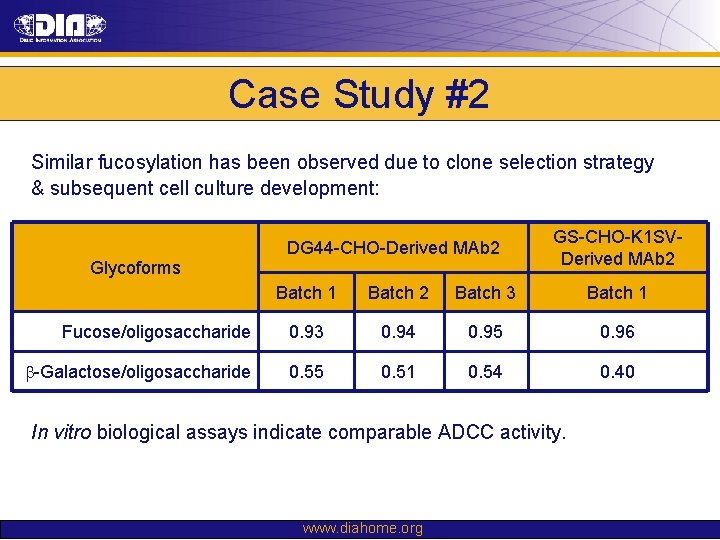
Case Study #2 Similar fucosylation has been observed due to clone selection strategy & subsequent cell culture development: Glycoforms DG 44 -CHO-Derived MAb 2 GS-CHO-K 1 SVDerived MAb 2 Batch 1 Batch 2 Batch 3 Batch 1 Fucose/oligosaccharide 0. 93 0. 94 0. 95 0. 96 b-Galactose/oligosaccharide 0. 55 0. 51 0. 54 0. 40 In vitro biological assays indicate comparable ADCC activity. www. diahome. org
Case Study #2 - Summary Physico-Chemical Testing: • No apparent adverse impact on structural integrity, product-related impurities or processrelated impurities • Minor differences observed in molecular heterogeneity – Lower b-galactosylation levels In vitro Biological Assays • No apparent differences observed in ADCC activity Nonclinical PK, PD & Immunogenicity Assessment • No apparent differences observed Thus far, cell line change presents low risk to the safety or efficacy of MAb 2 (manufacture of clinical trial lots is ongoing) www. diahome. org
Conclusions Characterization during clone selection can mitigate risks associated with a cell line change • Integrated comparability strategy for a cell line change should start prior to clone selection • MAb’s MOA & clonal variability in CQAs should drive clone selection strategy Cross-functional risk assessments play a critical role throughout; e. g. , • Defining CQAs for MAb • Defining clone selection strategy • Defining physico-chemical testing protocol & acceptance criteria • Defining need for & extent of nonclinical PK, PD & immunogenicity assessments • Assessing potential impact of observed differences When possible, comparability plan/protocol should be shared with FDA prior to execution (e. g. , briefing document, IND amendment) www. diahome. org
- Slides: 36