Essentials of Fire Fighting 6 th Edition Firefighter





































- Slides: 87

Essentials of Fire Fighting 6 th Edition Firefighter I Chapter 5 — Fire Behavior Replace with manual graphic on slide master

Learning Objective 1 Explain the science of fire as it relates to energy, forms of ignition, and modes of combustion. 5– 1

Understanding the physical science of fire can help firefighter safety. • Fire – Variety of forms • Heat-producing chemical reaction between fuel and oxidizer • Knowledge can help • Translate into practical knowledge of fire behavior • Recognize what is happening – Predict potential behavior 5– 2

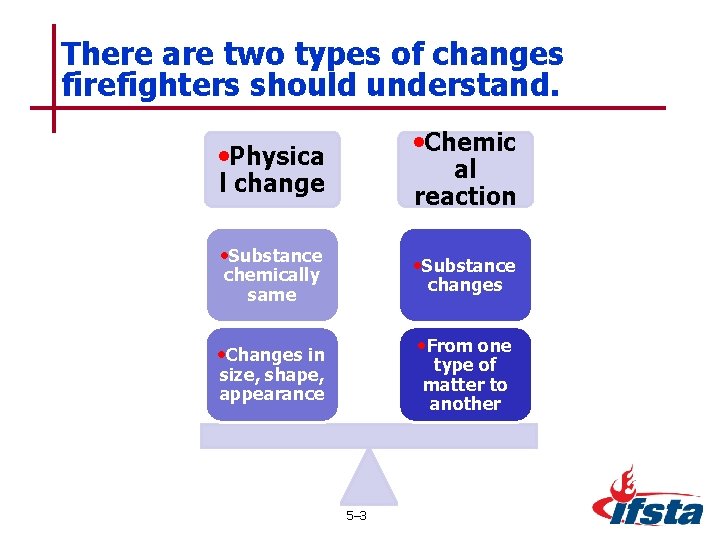
There are two types of changes firefighters should understand. • Chemic • Physica l change al reaction • Substance chemically same changes • From one • Changes in type of matter to another size, shape, appearance 5– 3

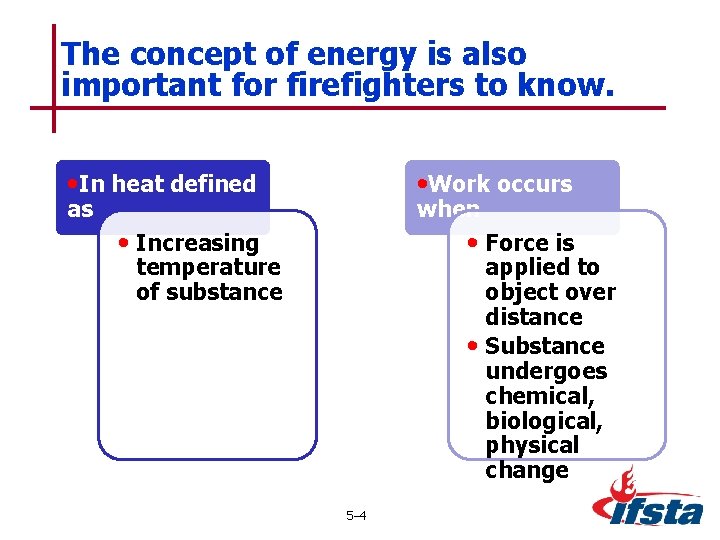
The concept of energy is also important for firefighters to know. • In heat defined • Work occurs • Increasing • Force is as when temperature of substance applied to object over distance • Substance undergoes chemical, biological, physical change 5– 4

There are two forms of energy that firefighters should know about. Courtesy of Dan Madrzykowski, NIST 5– 5

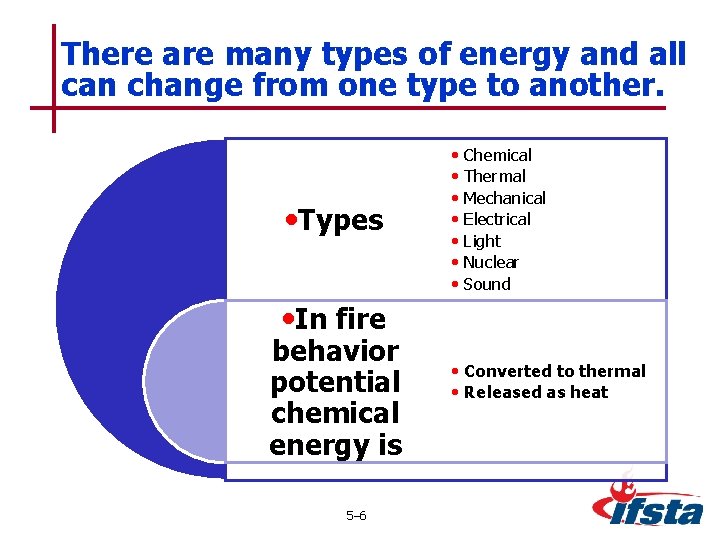
There are many types of energy and all can change from one type to another. • Types • Chemical • Thermal • Mechanical • Electrical • Light • Nuclear • Sound • In fire behavior potential chemical energy is 5– 6 • Converted to thermal • Released as heat

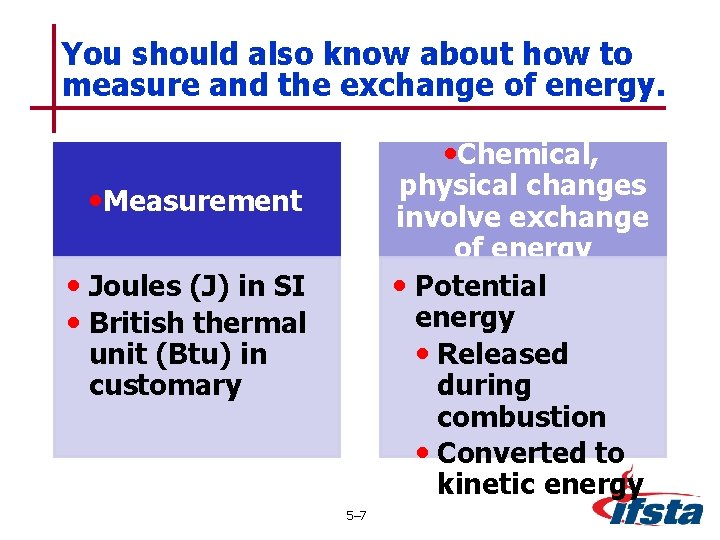
You should also know about how to measure and the exchange of energy. • Chemical, physical changes involve exchange of energy • Potential energy • Released during combustion • Converted to kinetic energy • Measurement • Joules (J) in SI • British thermal unit (Btu) in customary 5– 7

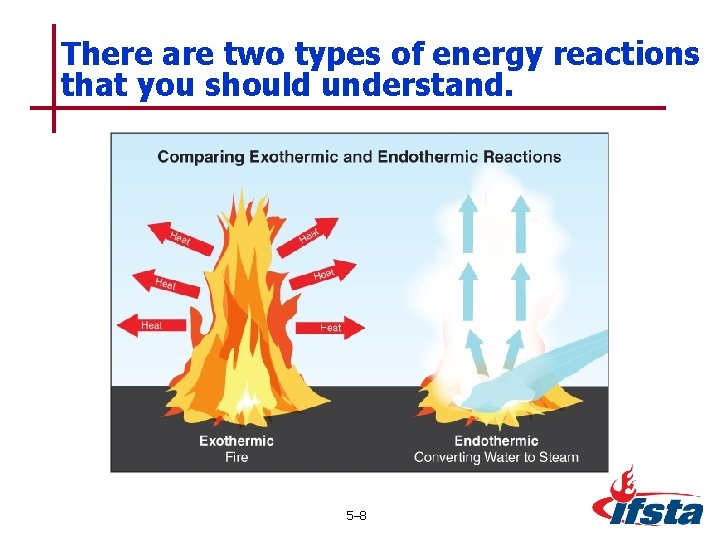
There are two types of energy reactions that you should understand. 5– 8

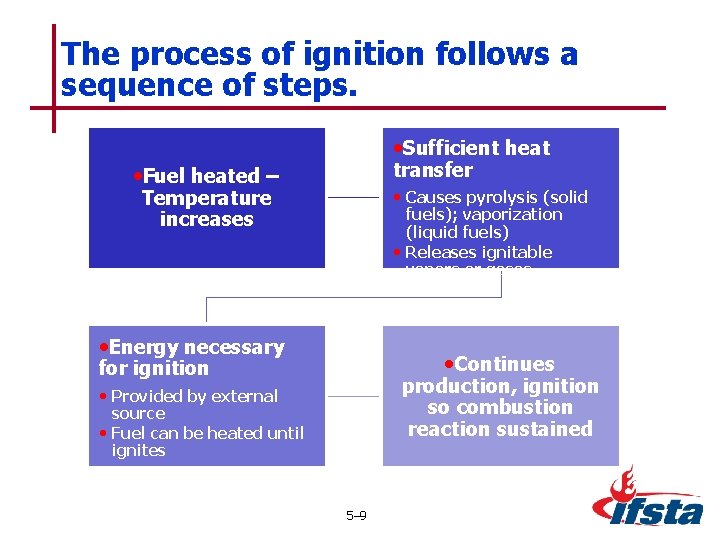
The process of ignition follows a sequence of steps. • Sufficient heat transfer • Fuel heated – Temperature increases • Causes pyrolysis (solid fuels); vaporization (liquid fuels) • Releases ignitable vapors or gases • Energy necessary • Continues for ignition production, ignition so combustion reaction sustained • Provided by external source • Fuel can be heated until ignites 5– 9

Piloted and autoignition are the two forms of ignition. 5– 10

Fire and combustion require similar conditions to occur. • Combustion – • Fire – One Chemical reaction, can occur without fire possible result of combustion 5– 11

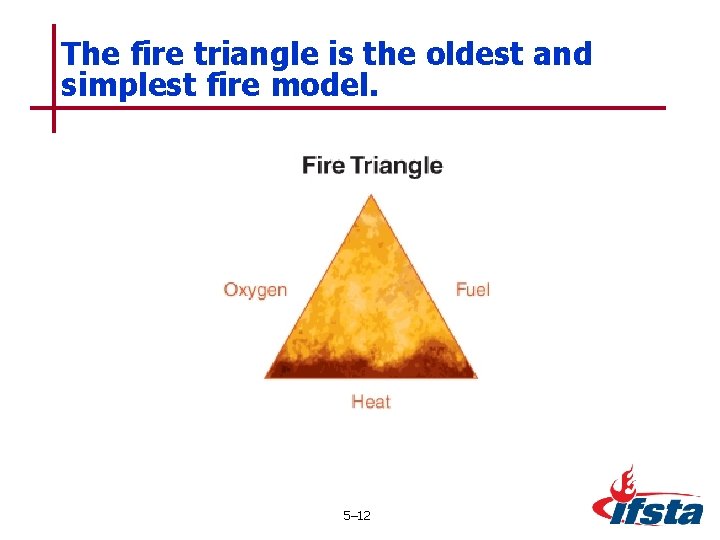
The fire triangle is the oldest and simplest fire model. 5– 12

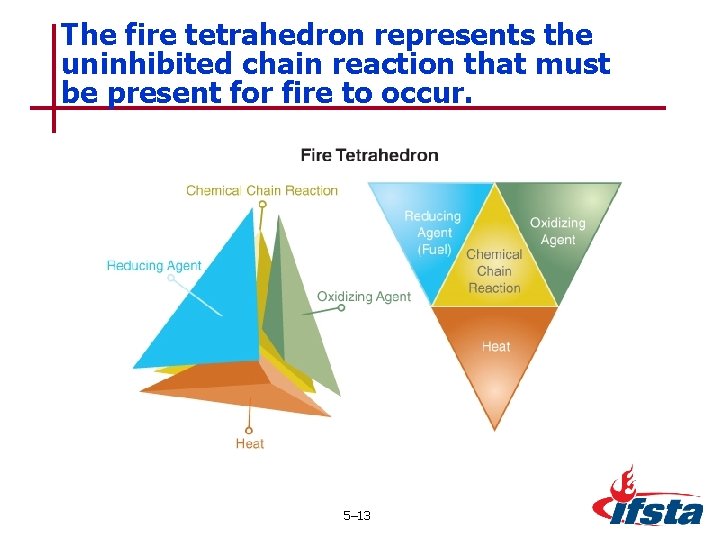
The fire tetrahedron represents the uninhibited chain reaction that must be present for fire to occur. 5– 13

There are several materials that affect both ignition and fire development. • Fuel • Passiv • Heat e agents • Oxyge n 5– 14

The two types of combustion occur under different circumstances. • Burning is localized on or near fuel’s surface – • Nonflaming Where in contact with oxygen 5– 15 • Gaseous fuel mixes with oxygen in correct ratio, heated to ignition temperature • Flamin

The products of combustion generate as fuel burns and changes chemical composition. • Thermal energy • Toxic smoke • Smoke (Cont. ) 5– 16

The products of combustion generate as fuel burns and changes chemical composition. • Carbon monoxide (CO) • Hydrogen cyanide (HCN) • Carbon dioxide (CO 2) (Cont. ) 5– 17

WARNING Smoke is fuel and is always potentially flammable. Wear full PPE and SCBA anytime you work in smoke. 5– 18

The products of combustion generate as fuel burns and changes chemical composition. • Flame 5– 19

REVIEW QUESTION How does the science of fire relate to energy, forms of ignition, and modes of combustion? 5– 20

Learning Objective 2 Describe the impact of thermal energy on heat, temperature, and heat transfer. 5– 21

Thermal energy (heat) is the energy element in both fire models. • Kinetic energy transfers from high-temperature to lowtemperature substance • Always in transit • Vibrates • Thermal kinetic molecules in fuel leading to break down, release of vapors needed to release potential chemical energy in fuel 5– 22

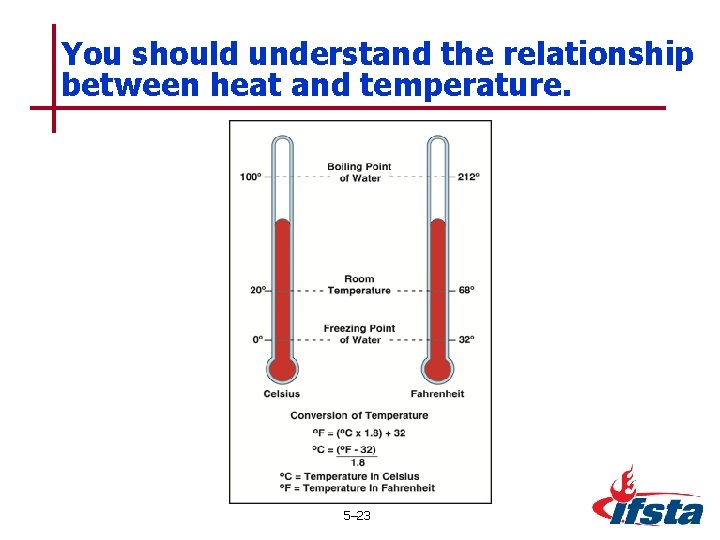
You should understand the relationship between heat and temperature. 5– 23

There are several sources of thermal energy you should recognize. • Chemica l • Electrica l • Mechani cal 5– 24 • Resistance heating • Overcurrent or overload • Arcing • Sparking

Understanding the concept of heat transfer can help in several ways. • Understand transfer from initial fuel package to others • Estimate size of fire before attacking – Evaluate effectiveness of attack • Transfer occurs from warmer to cooler – Same temperature cannot transfer 5– 25

The concept of transfer rate is influenced by several factors. • Related to temperature differential – Thermal conductivity • Greater temperature difference – Greater transfer rate • Heat flux 5– 26

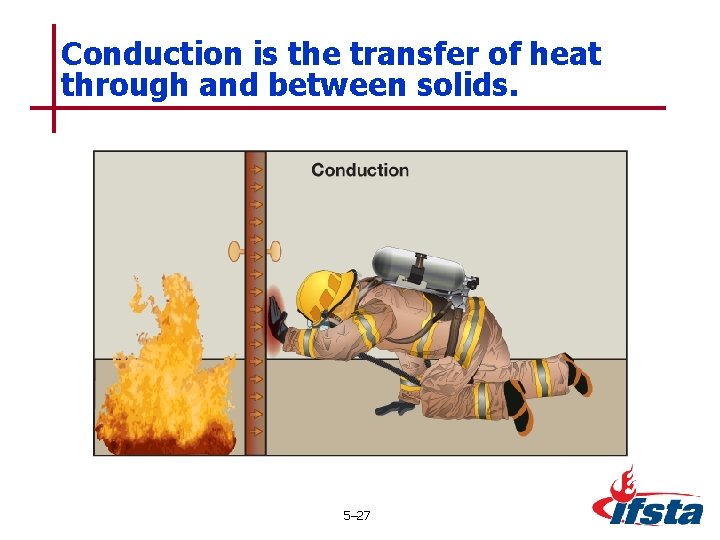
Conduction is the transfer of heat through and between solids. 5– 27

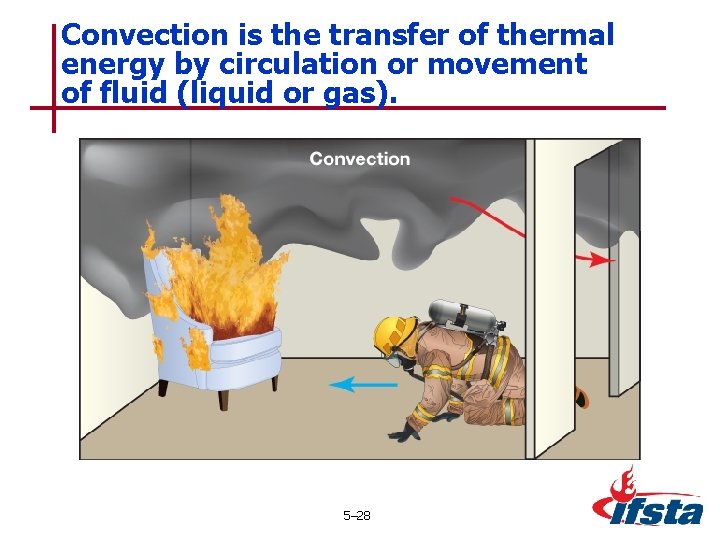
Convection is the transfer of thermal energy by circulation or movement of fluid (liquid or gas). 5– 28

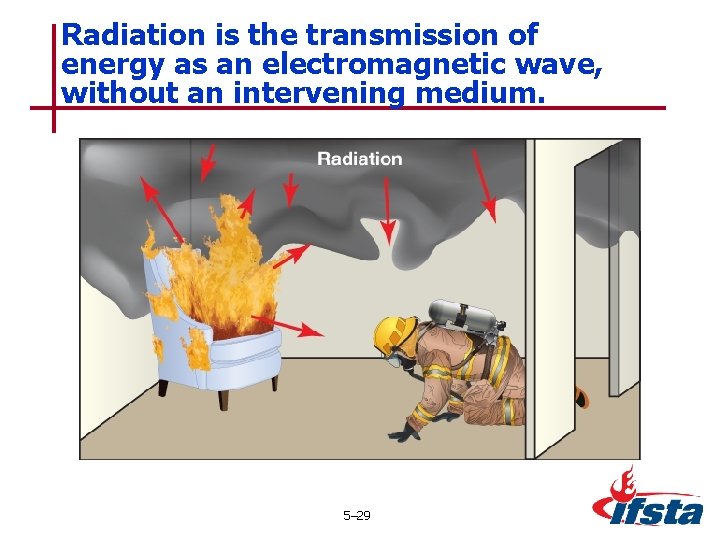
Radiation is the transmission of energy as an electromagnetic wave, without an intervening medium. 5– 29

REVIEW QUESTION What impact does thermal energy have on heat, temperature, and heat transfer? 5– 30

Learning Objective 3 Recognize the physical states of fuel. 5– 31

Fuel is the material or substance oxidized or burned in combustion. • Inorganic – Do not contain carbon • Organic – Contain carbon, other elements 5– 32

The chemical content of fuel influences heat of combustion and heat release rate. • Heat of combustion • Total amount of thermal energy released when specific amount of fuel oxidized (burned) • Heat release rates 5– 33

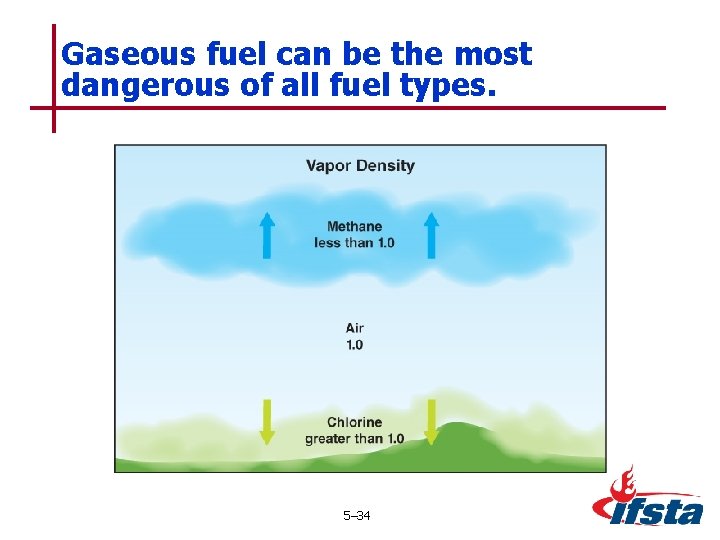
Gaseous fuel can be the most dangerous of all fuel types. 5– 34

The properties of liquid fuel are important to understand. • Mass, volume but no shape • Will not expand to fill all of container • Will flow downhill, can pool in low areas 5– 35

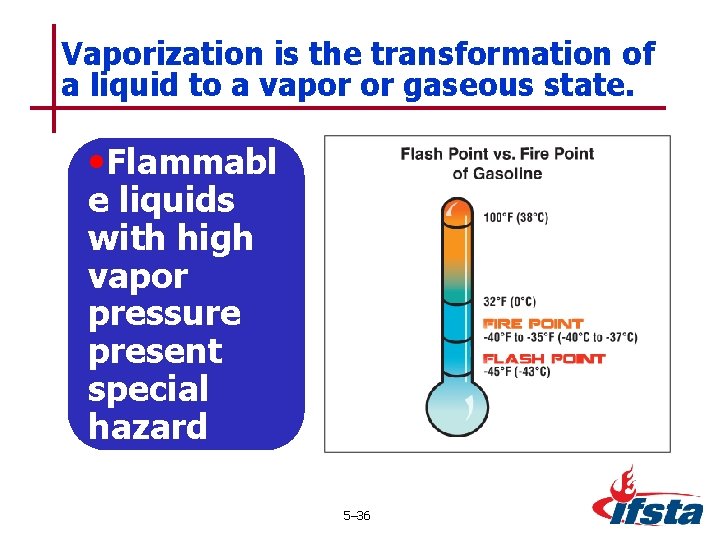
Vaporization is the transformation of a liquid to a vapor or gaseous state. • Flammabl e liquids with high vapor pressure present special hazard 5– 36

Solubility is a factor to consider regarding liquid fuels. • Solubili ty – • Miscible – Mix in any Extent to proportion which • Hydrocarbon – Do not mix substanc • Polar solvents – e will Readily mix with water 5– 37

Density is also a factor to consider regarding liquid fuels. • Liquids less dense than water difficult to extinguish with water alone • Water-soluble mix with agent – Become less effective • Fuel will not mix with water – Adding may disperse burning liquid • Extinguish with appropriate agent • Avoid use with foams specifically designed for polar solvents 5– 38

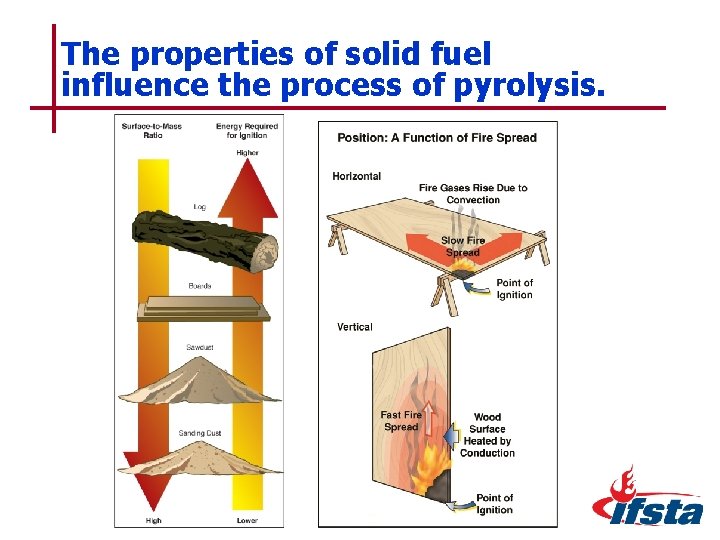
The properties of solid fuel influence the process of pyrolysis. 5– 39

REVIEW QUESTION What are the physical states that fuel can be found in? 5– 40

Learning Objective 4 Explain the relationship between oxygen and life safety. 5– 41

Oxygen is the primary oxidizing agent present at most fires. • At normal • 21 percent oxygen typical temperatures • Materials can ignite, burn at concentrations as low as 14 percent • Ambient • Limited oxygen diminishes flaming combustion • Higher oxygen temperature impacts concentrations than normal • Nonflaming • Flaming 5– 42

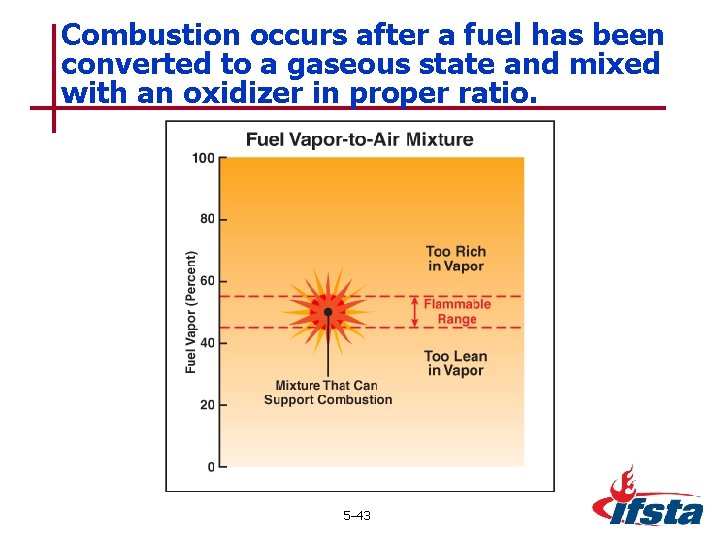
Combustion occurs after a fuel has been converted to a gaseous state and mixed with an oxidizer in proper ratio. 5– 43

REVIEW QUESTION How do oxygen and life safety relate to one another? 5– 44

Learning Objective 5 Identify the products of selfsustained chemical reactions. 5– 45

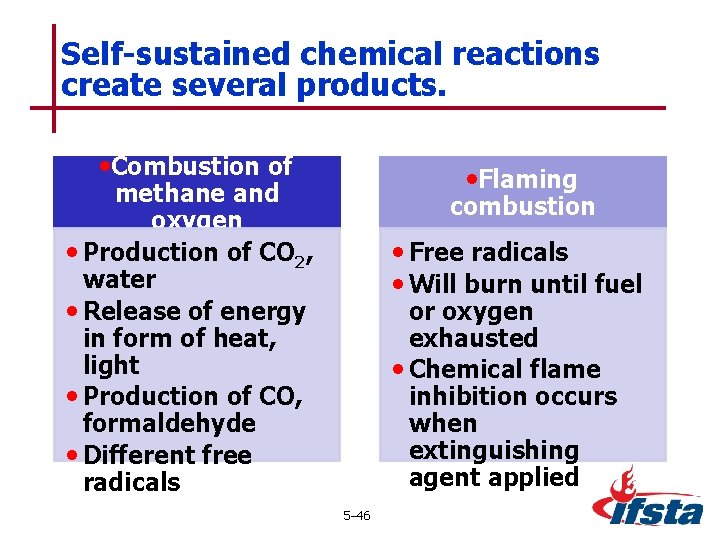
Self-sustained chemical reactions create several products. • Combustion of • Flaming methane and oxygen • Production of CO 2, water • Release of energy in form of heat, light • Production of CO, formaldehyde • Different free radicals combustion • Free radicals • Will burn until fuel or oxygen exhausted • Chemical flame inhibition occurs when extinguishing agent applied 5– 46

REVIEW QUESTION What products of self-sustained chemical reactions combine to make flammable and toxic substances? 5– 47

Learning Objective 6 Explain the factors that affect fire development. 5– 48

Learning Objective 7 Describe the stages of fire development. 5– 49

The stages of fire development occur in both unconfined and confined fires. Courtesy of Dan Madrzykowski, NIST Click image to play (Cont. ) Traditional – Lab development 5– 50

The stages of fire development occur in both unconfined and confined fires. Courtesy of Dan Madrzykowski, NIST Click image to play Actual – Real world development 5– 51

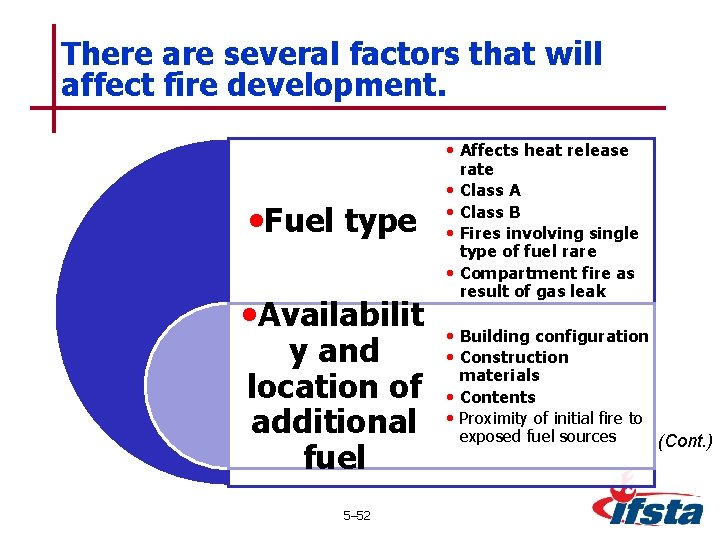
There are several factors that will affect fire development. • Affects heat release • Fuel type • Availabilit y and location of additional fuel 5– 52 rate • Class A • Class B • Fires involving single type of fuel rare • Compartment fire as result of gas leak • Building configuration • Construction materials • Contents • Proximity of initial fire to exposed fuel sources (Cont. )

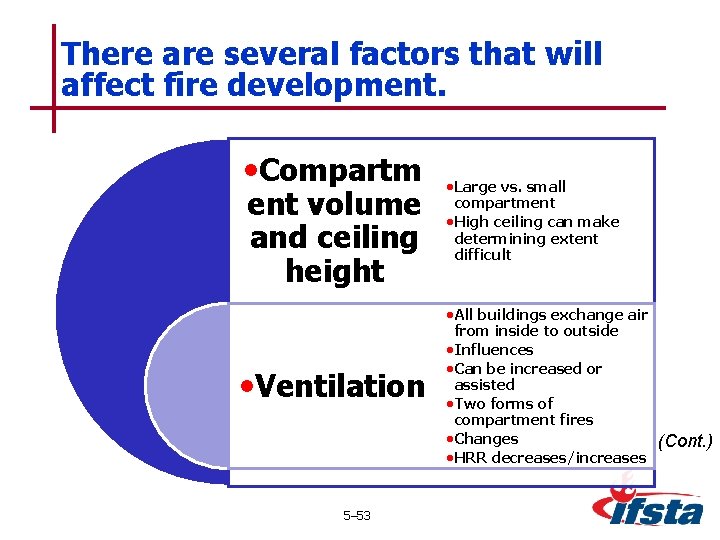
There are several factors that will affect fire development. • Compartm ent volume and ceiling height • Large vs. small compartment • High ceiling can make determining extent difficult • All buildings exchange air • Ventilation 5– 53 from inside to outside • Influences • Can be increased or assisted • Two forms of compartment fires • Changes • HRR decreases/increases (Cont. )

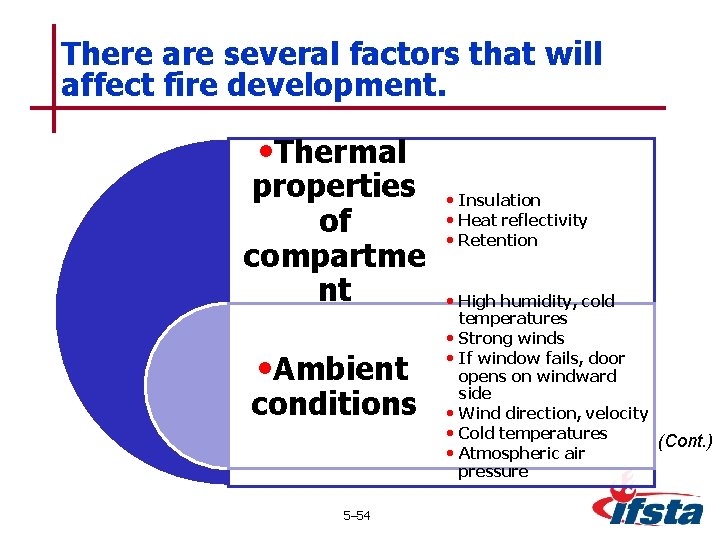
There are several factors that will affect fire development. • Thermal properties of compartme nt • Ambient conditions 5– 54 • Insulation • Heat reflectivity • Retention • High humidity, cold • • • temperatures Strong winds If window fails, door opens on windward side Wind direction, velocity Cold temperatures (Cont. ) Atmospheric air pressure

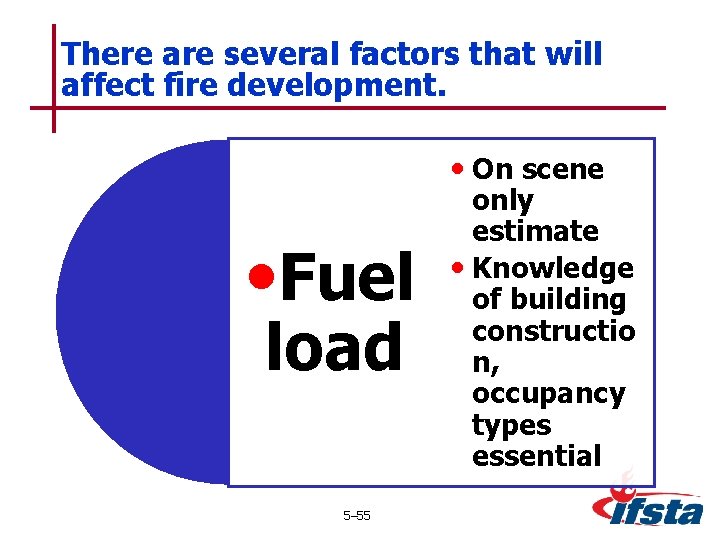
There are several factors that will affect fire development. • On scene • Fuel load 5– 55 only estimate • Knowledge of building constructio n, occupancy types essential

REVIEW QUESTION What different factors can impact fire development? 5– 56

The incipient stage starts when the elements of the fire triangle come together and combustion begins. Courtesy of Dan Madrzykowski, NIST 5– 57

The growth stage occurs as the fire transitions and is influenced by air in the compartment. Courtesy of Dan Madrzykowski, NIST 5– 58

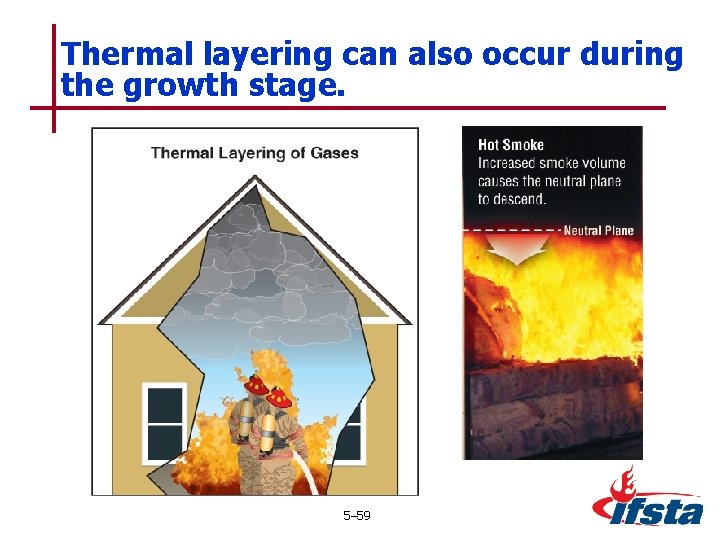
Thermal layering can also occur during the growth stage. 5– 59

Isolated flames and rapid transitions may also be a part of the growth stage. Courtesy of Dan Madrzykowski, NIST 5– 60

The fully developed stage occurs when all combustible materials are burning. Courtesy of Dan Madrzykowski, NIST 5– 61

The decay stage brings combustion to a complete stop through two means. Courtesy of Dan Madrzykowski, NIST 5– 62

REVIEW QUESTION What are the stages of fire development? 5– 63

Learning Objective 8 Recognize signs, causes, and effects of rapid fire development. 5– 64

Rapid fire development is responsible for numerous deaths and injuries. • Protect yourself and your crew • Recognize indicators • Know conditions created by • Determine best action to take before 5– 65

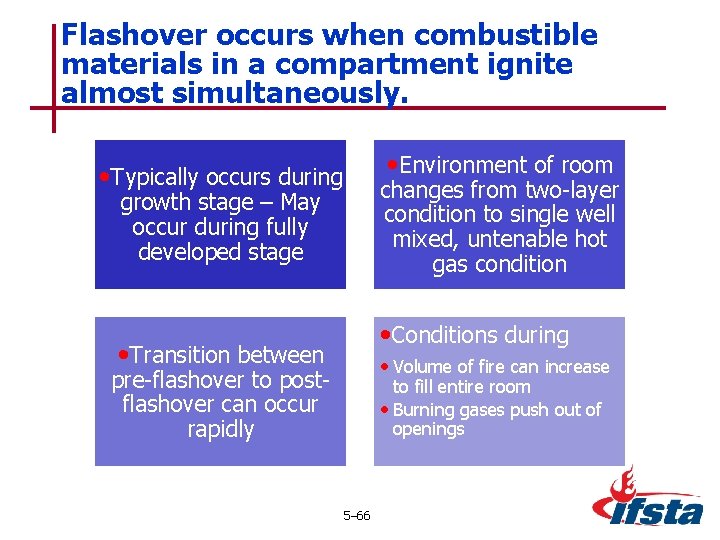
Flashover occurs when combustible materials in a compartment ignite almost simultaneously. • Environment of room • Typically occurs during changes from two-layer condition to single well mixed, untenable hot gas condition growth stage – May occur during fully developed stage • Conditions during • Transition between • Volume of fire can increase pre-flashover to postflashover can occur rapidly to fill entire room • Burning gases push out of openings 5– 66

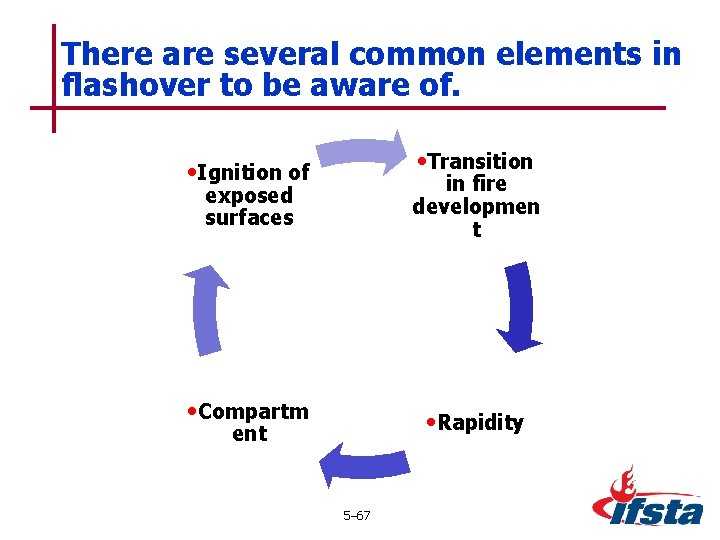
There are several common elements in flashover to be aware of. • Transition • Ignition of in fire developmen t exposed surfaces • Compartm • Rapidity ent 5– 67

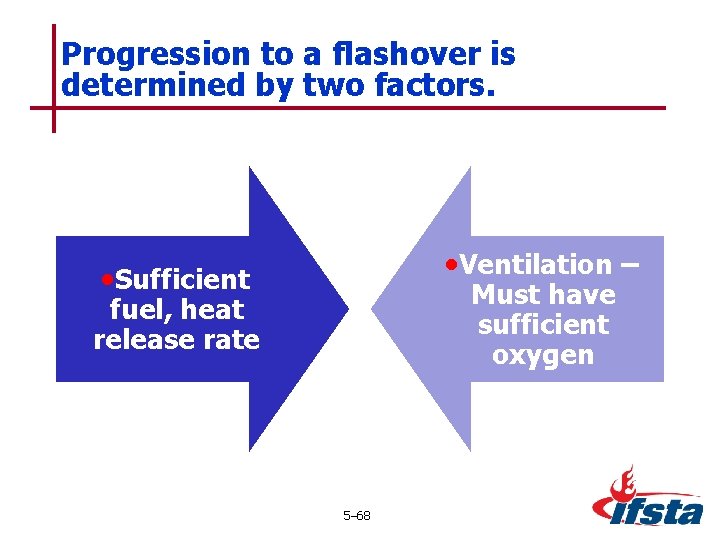
Progression to a flashover is determined by two factors. • Ventilation – • Sufficient Must have sufficient oxygen fuel, heat release rate 5– 68

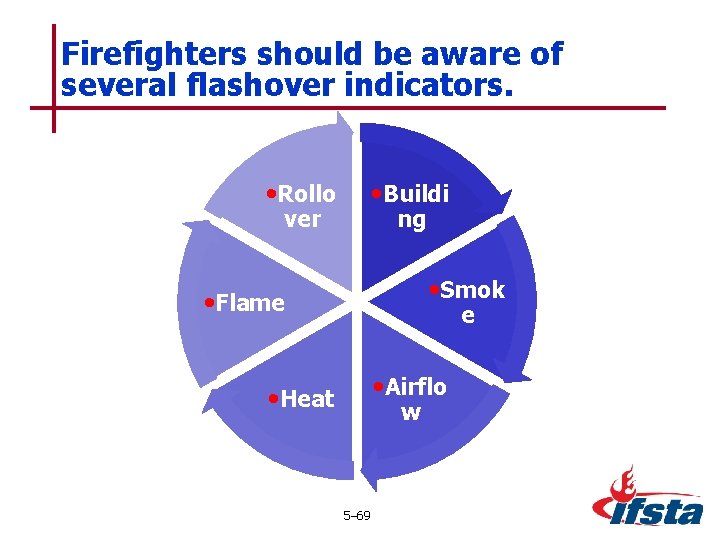
Firefighters should be aware of several flashover indicators. • Rollo • Buildi ver ng • Smok • Flame e • Airflo • Heat w 5– 69

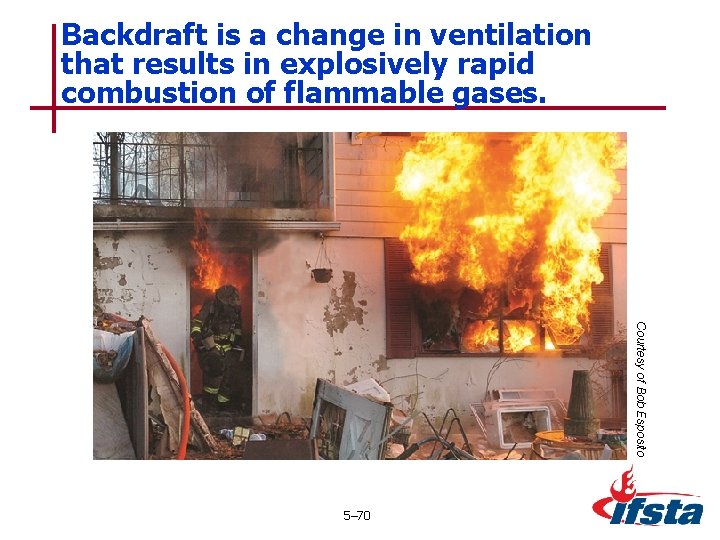
Backdraft is a change in ventilation that results in explosively rapid combustion of flammable gases. Courtesy of Bob Esposito 5– 70

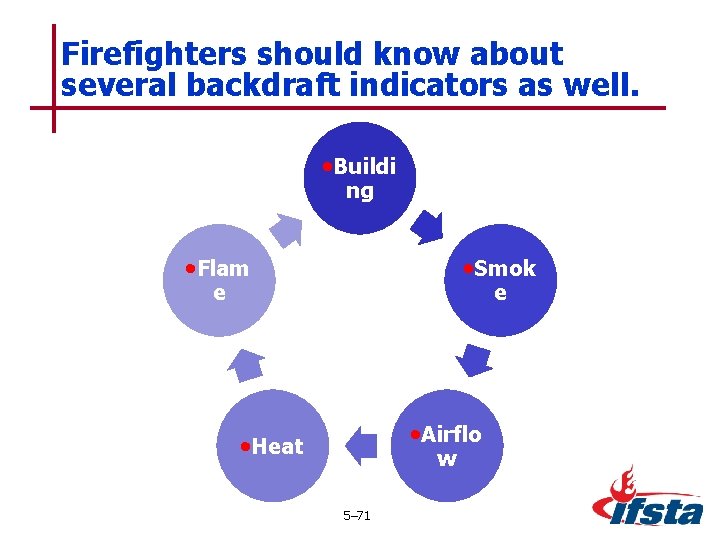
Firefighters should know about several backdraft indicators as well. • Buildi ng • Flam • Smok e e • Airflo • Heat w 5– 71

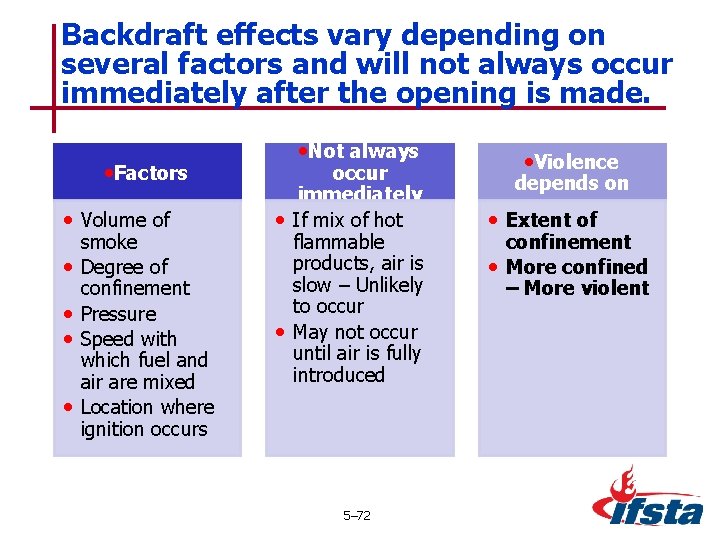
Backdraft effects vary depending on several factors and will not always occur immediately after the opening is made. • Factors • Volume of • • smoke Degree of confinement Pressure Speed with which fuel and air are mixed Location where ignition occurs • Not always occur immediately • If mix of hot flammable products, air is slow – Unlikely to occur • May not occur until air is fully introduced 5– 72 • Violence depends on • Extent of confinement • More confined – More violent

A smoke explosion may occur before or after the decay stage as unburned fuel gases contact an ignition source. • Cooling smoke can accumulate in other areas, mix with air • Violent because involve premixed fuel, oxygen 5– 73 • Smoke • Generally cool – Less than 1, 112 o F (600 o C) • Located in void spaces or uninvolved areas

REVIEW QUESTION What are the signs and causes of a backdraft? 5– 74

Learning Objective 9 Describe the methods through which fire fighting operations can influence fire behavior. 5– 75

Firefighters can influence fire behavior through temperature reduction. • Water used • Cooling with water most common method • Water has to control burning gases, reduce temperature of products of combustion greatest effect when converted to steam 5– 76 • Control steam production

Firefighters can influence fire behavior through fuel removal. • Simplest – Allow to burn until all is consumed • May allow fire to burn – Minimize groundwater pollution 5– 77 • Other methods

Oxygen exclusion reduces a fire’s growth and may extinguish it over time. • Closing doors • Methods – Will can limit air supply, help prevent flashover not work if fuel is self-oxidizing 5– 78

Chemical flame inhibition uses agents to interrupt the combustion reaction. • Effective on gas, liquid fuels • Do not easily extinguish nonflaming fires 5– 79 • Not practical for smoldering fires

Unplanned ventilation may occur before or after suppression operations start. • Can be result wind outside structure • Increase pressure inside structure • Drive smoke, flames into unburned portions • Upset tactical ventilation (Cont. ) 5– 80

WARNING Wind driven conditions can occur in any type of structure. Wind speeds as low as 10 mph (16 kph) can create wind-driven conditions. 5– 81

Unplanned ventilation may occur before or after suppression operations start. • May be result of • Occupant action • Fire effects on building • Action outside of planned ventilation 5– 82

Tactical ventilation is planned, systematic, and coordinated. • Influence • Must be coordinated with suppression operations behavior based on • HHR increased when ventilation increased • Can be simple or complex 5– 83 • Increase in combustion rate when controlled

WARNING Even coordinated tactical ventilation increases the combustion rate in ventilation controlled fires. 5– 84

REVIEW QUESTION How can fire fighting operations impact fire behavior? 5– 85

Summary • You need to understand the combustion process, how fire behaves, and how to select appropriate extinguishing agents. • Understanding fire behavior can help you recognize developing fire conditions and respond safely to mitigate hazards present in the fire environment. 5– 86
 Firefighter essentials 7th edition
Firefighter essentials 7th edition Impulse momentum bar chart
Impulse momentum bar chart Fire suffix
Fire suffix Basic fire fighting training ppt
Basic fire fighting training ppt Streams of fire
Streams of fire Fire sprinkler head gpm chart
Fire sprinkler head gpm chart Bounce back
Bounce back Negative pressure ventilation firefighting
Negative pressure ventilation firefighting Ship fire fighting equipment
Ship fire fighting equipment Method of fire fighting
Method of fire fighting What is starvation in fire fighting
What is starvation in fire fighting Ubbl selangor
Ubbl selangor Rotary control valve firefighting
Rotary control valve firefighting Nstm 555 2021
Nstm 555 2021 Foam pro 1600
Foam pro 1600 Shipboard fire fighting organization
Shipboard fire fighting organization Nitin group
Nitin group At a bulk transport incident firefighters must
At a bulk transport incident firefighters must Essentials of mis, 13th edition
Essentials of mis, 13th edition Business essentials 12th edition answer key
Business essentials 12th edition answer key Essentials of organizational behavior 14th edition
Essentials of organizational behavior 14th edition William stallings network security essentials 5th edition
William stallings network security essentials 5th edition Essentials of sociology 5th edition
Essentials of sociology 5th edition Cryptographic systems are generically classified by
Cryptographic systems are generically classified by Business essentials 12th edition chapter 1
Business essentials 12th edition chapter 1 Business essentials 12th edition
Business essentials 12th edition Nonintervention perspective of criminal justice
Nonintervention perspective of criminal justice Essentials of investments 11th edition
Essentials of investments 11th edition Mis chapter 6
Mis chapter 6 Zulily case study
Zulily case study A gift of fire 4th edition
A gift of fire 4th edition A gift of fire 4th edition
A gift of fire 4th edition This needed three or more rescuers to form a hammock
This needed three or more rescuers to form a hammock Solid beam ground ladder
Solid beam ground ladder Nfpa lesson plans
Nfpa lesson plans Grab lives firefighter acronym
Grab lives firefighter acronym Firefighter support foundation
Firefighter support foundation Firefighter hose
Firefighter hose Pqa interview
Pqa interview Parts of ladder
Parts of ladder Firefighter
Firefighter Dmv firefighter endorsement test
Dmv firefighter endorsement test Lafd interview
Lafd interview Firefighter maze plans
Firefighter maze plans Vertical ventilation definition
Vertical ventilation definition Mine rescue 13-3
Mine rescue 13-3 Reverse horseshoe hose load
Reverse horseshoe hose load Alarm
Alarm Fire hose reel signage standards
Fire hose reel signage standards Ire fire fire rwi
Ire fire fire rwi Reichstag fire who was the fire starter
Reichstag fire who was the fire starter Fight fire with fire definition
Fight fire with fire definition God is fighting for us pushing back the darkness
God is fighting for us pushing back the darkness Telenor kontaktcenter
Telenor kontaktcenter Fighting taking on
Fighting taking on Kite flying in afghanistan
Kite flying in afghanistan Fighting position reference
Fighting position reference Fighting hartebees type of interaction
Fighting hartebees type of interaction The civil war the fighting escalates
The civil war the fighting escalates What does the nurse give to romeo from juliet?
What does the nurse give to romeo from juliet? Vietnam fighting style
Vietnam fighting style Fighting hunger
Fighting hunger Mrs foul
Mrs foul The history of kites
The history of kites Bushido fighting style
Bushido fighting style Strategic management a dynamic perspective
Strategic management a dynamic perspective Israeli fighting system
Israeli fighting system Armoured fighting vehicle
Armoured fighting vehicle Fighting terrorism
Fighting terrorism Fighting inflation means on
Fighting inflation means on A navy diver is not a fighting man
A navy diver is not a fighting man The interlopers summary
The interlopers summary Israeli fighting system
Israeli fighting system Polish people fighting
Polish people fighting Chapter 40 fighting the cold war at home
Chapter 40 fighting the cold war at home Fighting l
Fighting l About what are proctor and putnam fighting?
About what are proctor and putnam fighting? F-14 vs f-16
F-14 vs f-16 Marketing essentials chapter 2
Marketing essentials chapter 2 What are the ten essentials of a successful ffa chapter
What are the ten essentials of a successful ffa chapter Chapter 13 initiating the sale
Chapter 13 initiating the sale Marketing essentials chapter 38
Marketing essentials chapter 38 Windows 2019 essentials limits
Windows 2019 essentials limits What element creates the tone and presents the characters
What element creates the tone and presents the characters Character strong 8 essentials
Character strong 8 essentials Essentials of technical communication
Essentials of technical communication What are the ten essentials of a successful ffa chapter?
What are the ten essentials of a successful ffa chapter? Cash budget benefits
Cash budget benefits