Essentials of Biology Sylvia S Mader Chapter 2

Essentials of Biology Sylvia S. Mader Chapter 2 Lecture Outline Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

2. 1 The Nature of Matter § § § Anything that takes up space and has mass Can exist as a solid, liquid, or a gas Composed of elements • Element § § • • cannot be broken down into another substance by ordinary chemical means Pure substance consisting of one type of atom Only 92 naturally occurring elements Four most common elements in living organisms – CHON
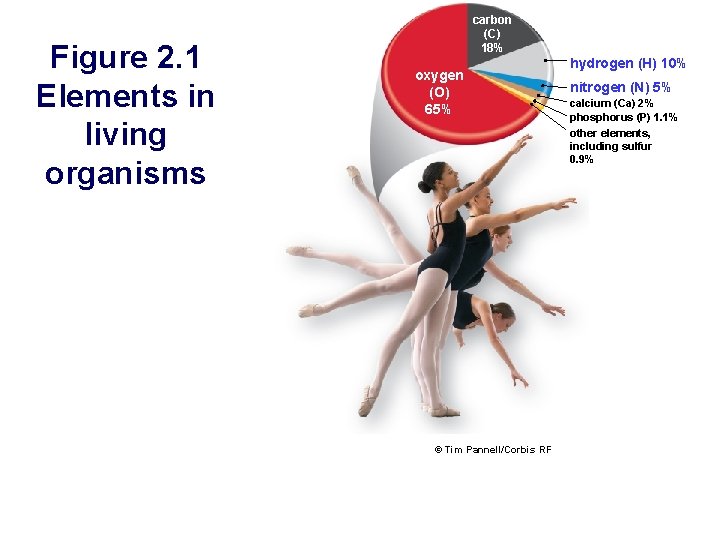
Figure 2. 1 Elements in living organisms carbon (C) 18% oxygen (O) 65% © Tim Pannell/Corbis RF hydrogen (H) 10% nitrogen (N) 5% calcium (Ca) 2% phosphorus (P) 1. 1% other elements, including sulfur 0. 9%
Atomic structure § Atomic theory • Elements consist of atoms • Atom: smallest unit of an element that has the properties of the element § Atomic symbols – one or two letters • H=? C=? S= ? Cl = ? Na = ? § Subatomic particles • Neutrons, n § no electrical charge, found in nucleus • Protons, p+ § positive charge, found in nucleus § determines the ID of an atom • Electrons , e§ negative charge, outside of nucleus § determines the chemical properties of an atom § Mass number • sum of protons and neutrons (electrons have nearly zero mass)
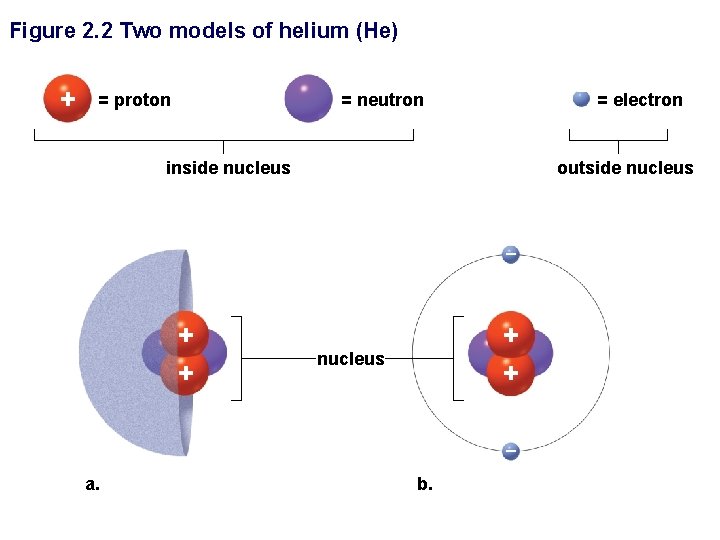
Figure 2. 2 Two models of helium (He) + = proton – = neutron inside nucleus outside nucleus – + + nucleus – a. = electron b.
Atomic number § All atoms of an element have this same number of protons. § Atoms are electrically neutral: • How do the number of protons compare to the number of electrons? • Periodic table § Elements in order of their _________. § Atoms arranged in periods (rows) and groups (columns) § Elements’ chemical and physical characteristics recur in predictable manner • E. g. Li. Cl, Na. Cl, KCl; Be. Cl 2, Ca. Cl? ? ?
Periodic table of the elements Location of. . • Metals? • Nonmetals?
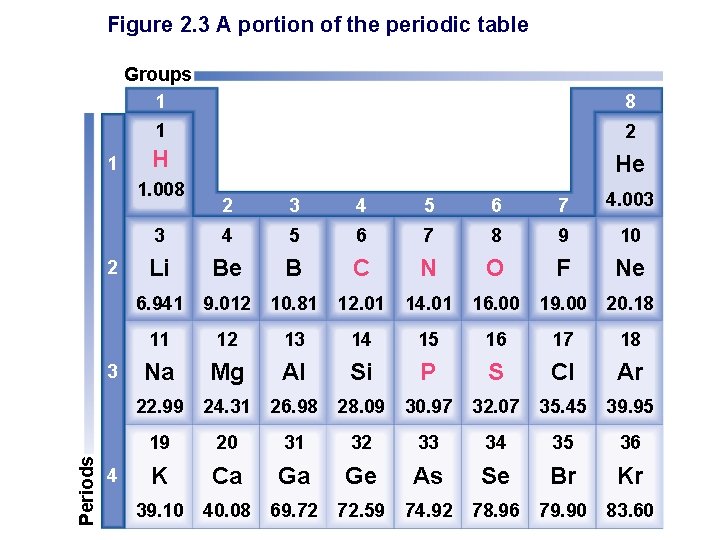
Figure 2. 3 A portion of the periodic table 1 Groups 1 8 1 2 H He 1. 008 2 Periods 3 4 2 3 4 5 6 7 4. 003 3 4 5 6 7 8 9 10 Li Be B C N O F Ne 6. 941 9. 012 10. 81 12. 01 14. 01 16. 00 19. 00 20. 18 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar 22. 99 24. 31 26. 98 28. 09 30. 97 32. 07 35. 45 39. 95 19 20 31 32 33 34 35 36 K Ca Ga Ge As Se Br Kr 39. 10 40. 08 69. 72 72. 59 74. 92 78. 96 79. 90 83. 60

Isotopes § Atoms of the same element that differ in the number of _________. § Isotopes have the same number of ____ but a different number of ______ (different mass numbers) § Unstable isotopes may decay emitting radiation § Radioactive isotopes used… • Can be used as tracer – PET scan • Can cause damage to cells leading to cancer • Can be used to sterilize medical equipment
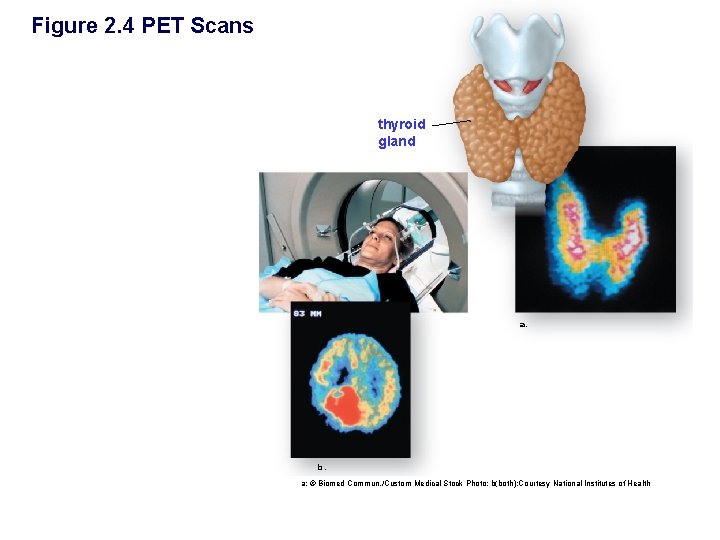
Figure 2. 4 PET Scans thyroid gland a. b. a: © Biomed Commun. /Custom Medical Stock Photo; b(both): Courtesy National Institutes of Health
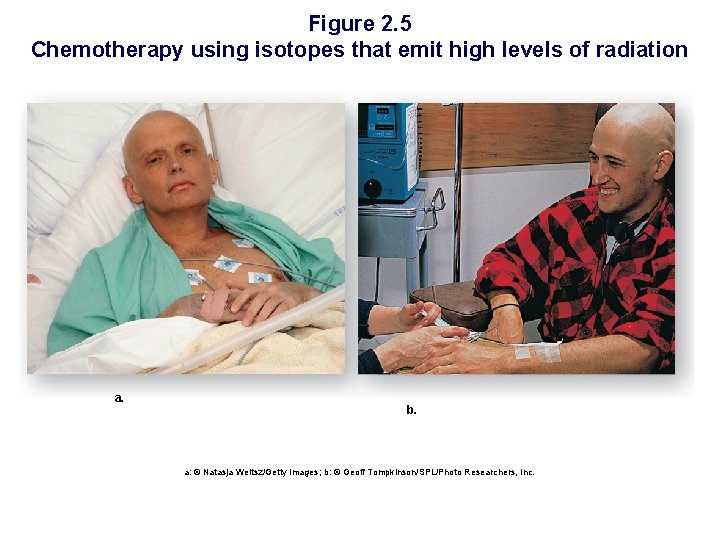
Figure 2. 5 Chemotherapy using isotopes that emit high levels of radiation a. b. a: © Natasja Weitsz/Getty Images; b: © Geoff Tompkinson/SPL/Photo Researchers, Inc.

Arrangements of Electrons § Located outside the nucleus of an atom in specific electron shells (energy levels) § Each shell contains a certain number of electrons § For atoms up through number 20 • 2 electrons fill first shell. • 8 electrons fill each additional shell. § Octet rule for valence shell • Valence shell: outermost shell • Atoms are most stable with 8 valence electrons. § Exceptions: atoms with the only one energy level • Atoms can give up, accept, or share electrons to have 8. § The number of electrons in the valence shell determines the chemical properties of an atom
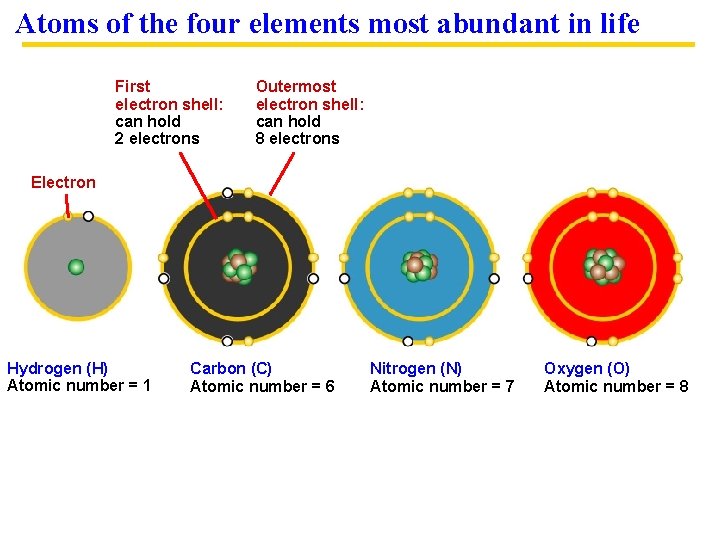
Atoms of the four elements most abundant in life First electron shell: can hold 2 electrons Outermost electron shell: can hold 8 electrons Electron Hydrogen (H) Atomic number = 1 Carbon (C) Atomic number = 6 Nitrogen (N) Atomic number = 7 Oxygen (O) Atomic number = 8
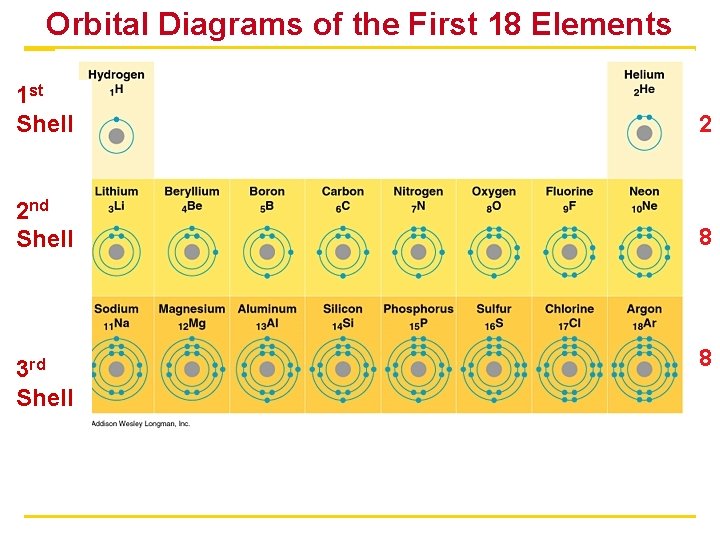
Orbital Diagrams of the First 18 Elements 1 st Shell 2 2 nd Shell 8 3 rd Shell 8
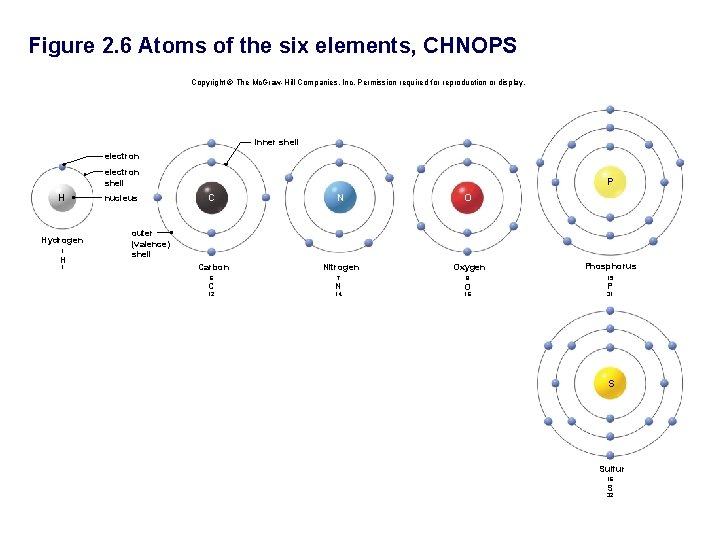
Figure 2. 6 Atoms of the six elements, CHNOPS Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. inner shell electron shell H Hydrogen 1 H 1 nucleus P C N O Carbon Nitrogen outer (valence) shell Oxygen Phosphorus 6 7 8 15 C N O 12 14 16 P 31 S Sulfur 16 S 32
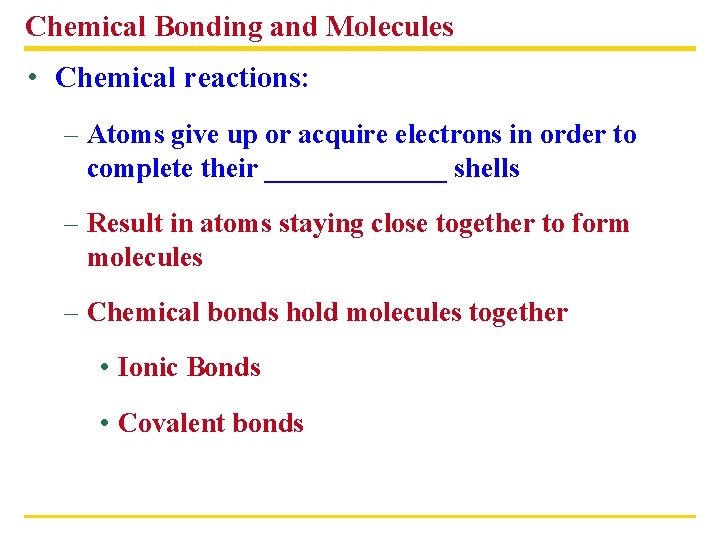
Chemical Bonding and Molecules • Chemical reactions: – Atoms give up or acquire electrons in order to complete their _______ shells – Result in atoms staying close together to form molecules – Chemical bonds hold molecules together • Ionic Bonds • Covalent bonds
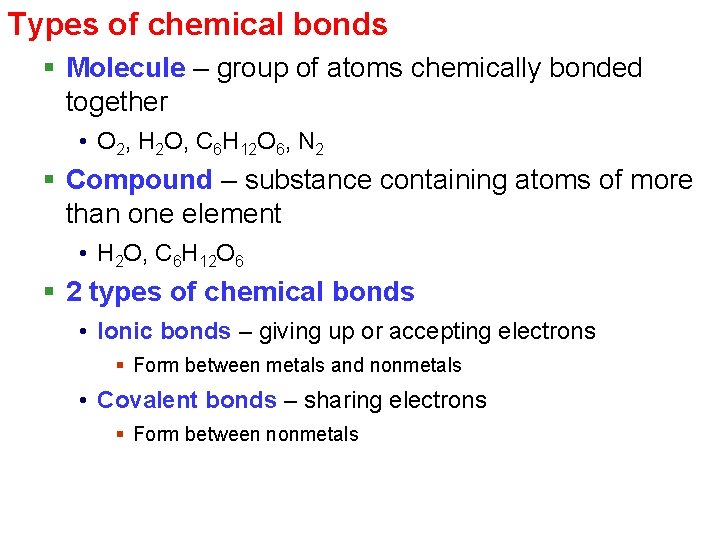
Types of chemical bonds § Molecule – group of atoms chemically bonded together • O 2, H 2 O, C 6 H 12 O 6, N 2 § Compound – substance containing atoms of more than one element • H 2 O, C 6 H 12 O 6 § 2 types of chemical bonds • Ionic bonds – giving up or accepting electrons § Form between metals and nonmetals • Covalent bonds – sharing electrons § Form between nonmetals
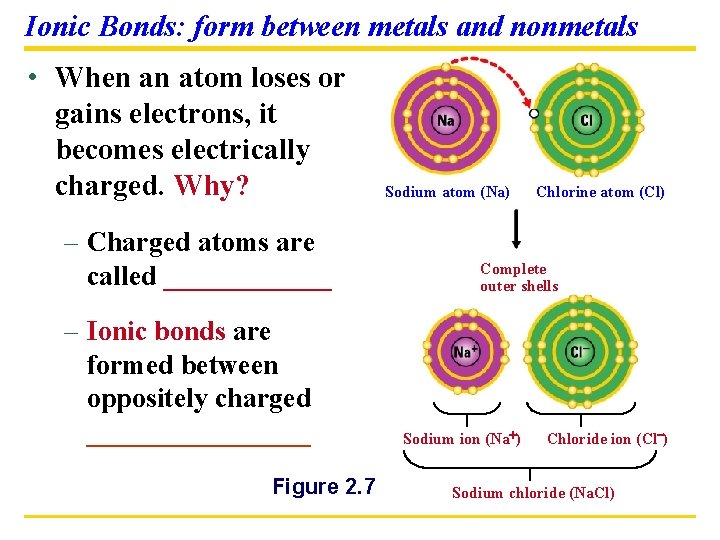
Ionic Bonds: form between metals and nonmetals • When an atom loses or gains electrons, it becomes electrically charged. Why? – Charged atoms are called ______ – Ionic bonds are formed between oppositely charged ________ Figure 2. 7 Sodium atom (Na) Chlorine atom (Cl) Complete outer shells Sodium ion (Na ) Chloride ion (Cl ) Sodium chloride (Na. Cl)
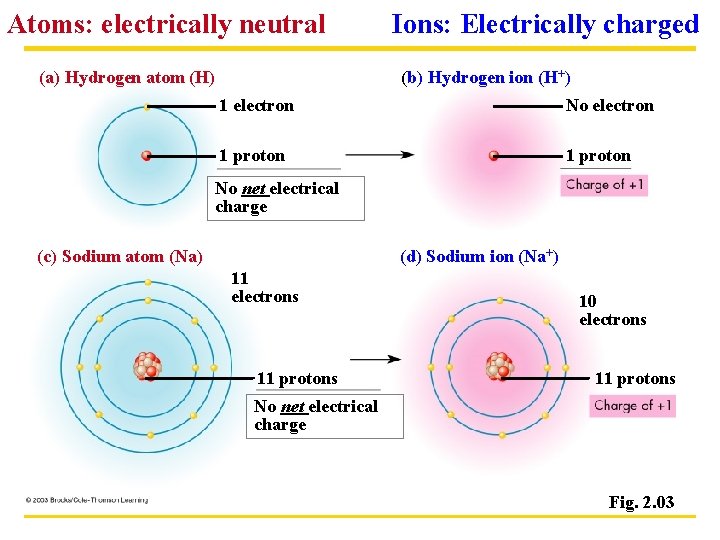
Atoms: electrically neutral (a) Hydrogen atom (H) Ions: Electrically charged (b) Hydrogen ion (H+) 1 electron No electron 1 proton No net electrical charge (c) Sodium atom (Na) (d) Sodium ion (Na+) 11 electrons 11 protons 10 electrons 11 protons No net electrical charge Fig. 2. 03
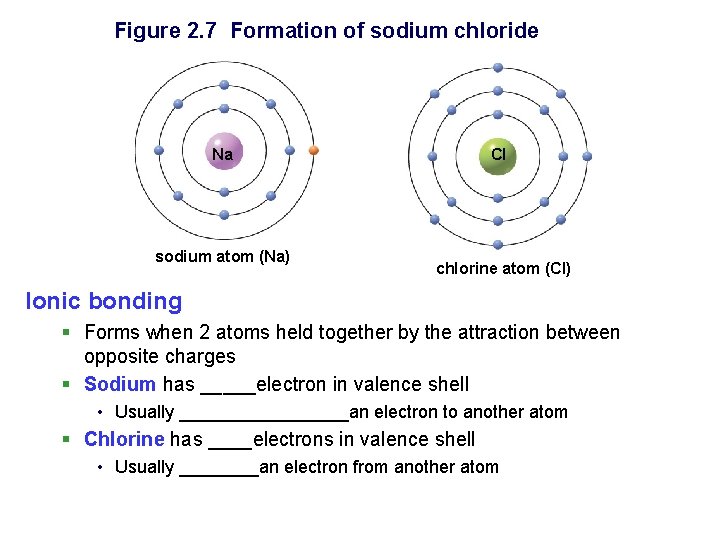
Figure 2. 7 Formation of sodium chloride Na sodium atom (Na) Cl chlorine atom (Cl) Ionic bonding § Forms when 2 atoms held together by the attraction between opposite charges § Sodium has _____electron in valence shell • Usually _________an electron to another atom § Chlorine has ____electrons in valence shell • Usually ____an electron from another atom
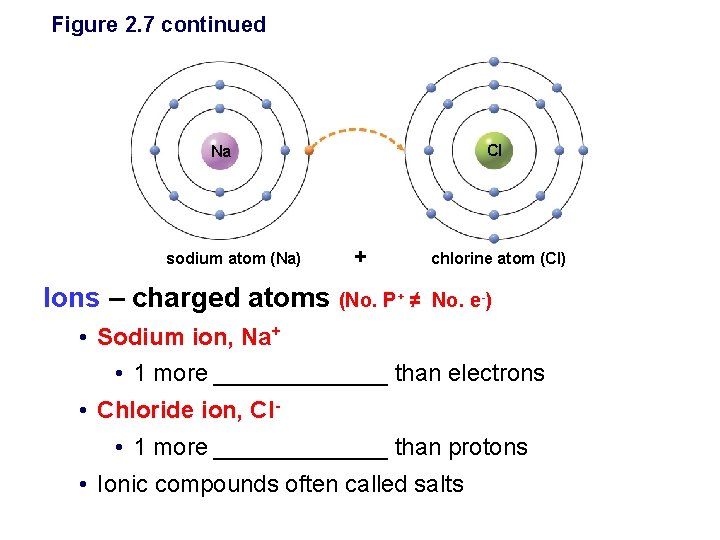
Figure 2. 7 continued Cl Na sodium atom (Na) + Ions – charged atoms (No. P+ ≠ chlorine atom (Cl) No. e-) • Sodium ion, Na+ • 1 more _______ than electrons • Chloride ion, Cl • 1 more _______ than protons • Ionic compounds often called salts
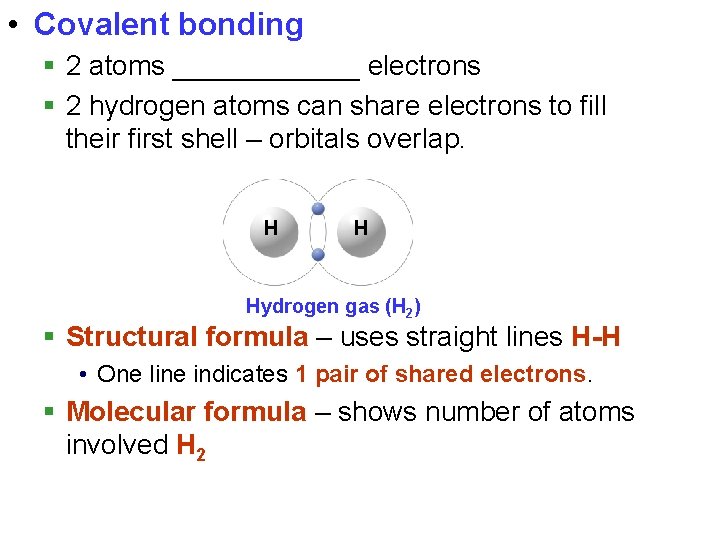
• Covalent bonding § 2 atoms ______ electrons § 2 hydrogen atoms can share electrons to fill their first shell – orbitals overlap. H H Hydrogen gas (H 2) § Structural formula – uses straight lines H-H • One line indicates 1 pair of shared electrons. § Molecular formula – shows number of atoms involved H 2
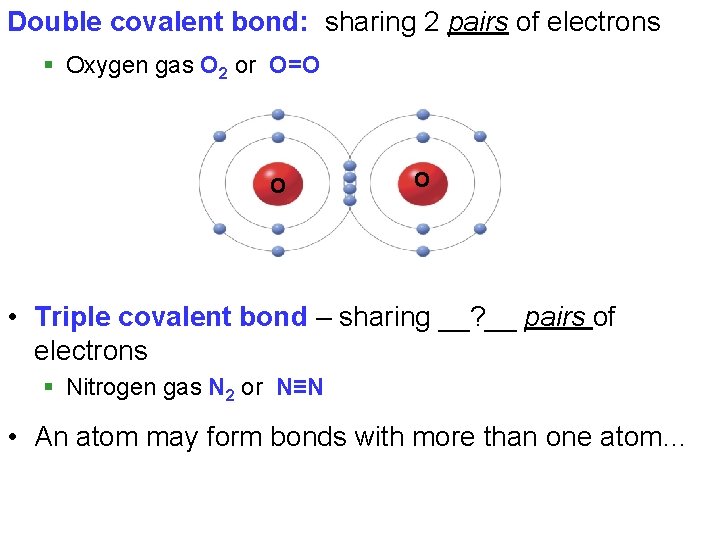
Double covalent bond: sharing 2 pairs of electrons § Oxygen gas O 2 or O=O O O • Triple covalent bond – sharing __? __ pairs of electrons § Nitrogen gas N 2 or N≡N • An atom may form bonds with more than one atom…
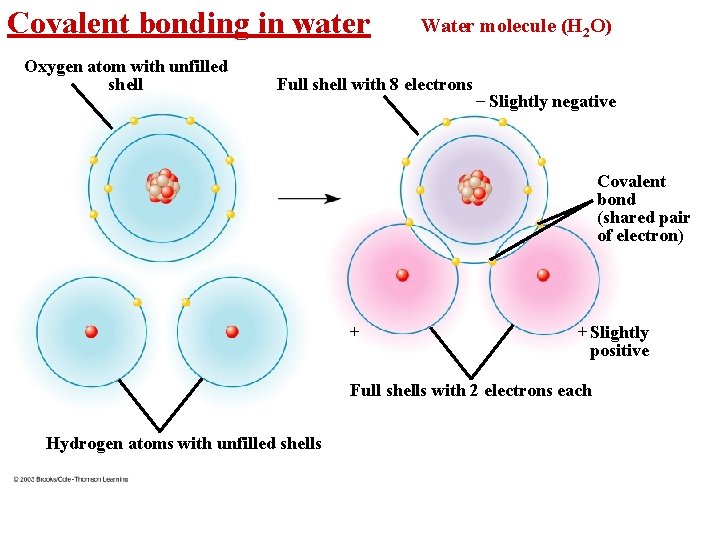
Covalent bonding in water Oxygen atom with unfilled shell Water molecule (H 2 O) Full shell with 8 electrons – Slightly negative Covalent bond (shared pair of electron) + + Slightly positive Full shells with 2 electrons each Hydrogen atoms with unfilled shells
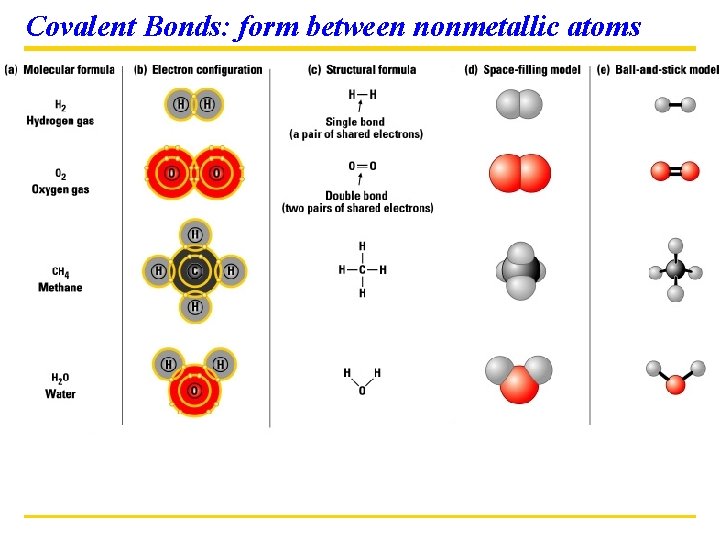
Covalent Bonds: form between nonmetallic atoms
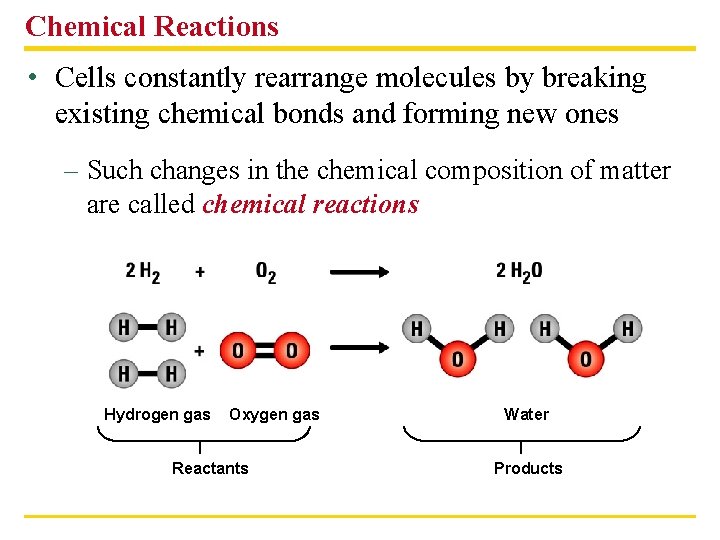
Chemical Reactions • Cells constantly rearrange molecules by breaking existing chemical bonds and forming new ones – Such changes in the chemical composition of matter are called chemical reactions Hydrogen gas Oxygen gas Reactants Water Products
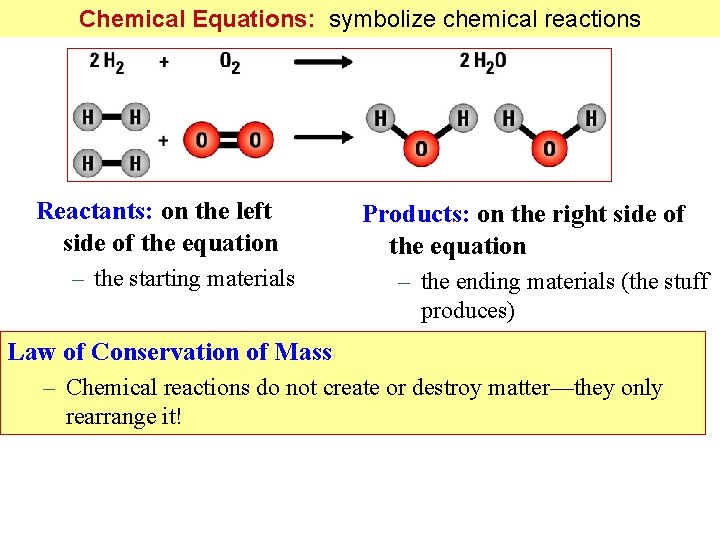
Chemical Equations: symbolize chemical reactions Reactants: on the left side of the equation – the starting materials Products: on the right side of the equation – the ending materials (the stuff produces) Law of Conservation of Mass – Chemical reactions do not create or destroy matter—they only rearrange it!
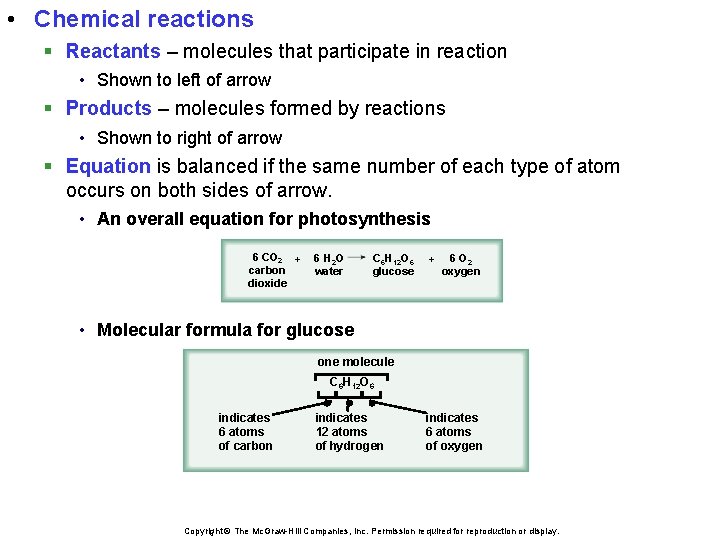
• Chemical reactions § Reactants – molecules that participate in reaction • Shown to left of arrow § Products – molecules formed by reactions • Shown to right of arrow § Equation is balanced if the same number of each type of atom occurs on both sides of arrow. • An overall equation for photosynthesis 6 CO 2 + carbon dioxide 6 H 2 O water C 6 H 12 O 6 glucose + 6 O 2 oxygen • Molecular formula for glucose one molecule C 6 H 12 O 6 indicates 6 atoms of carbon indicates 12 atoms of hydrogen indicates 6 atoms of oxygen Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.
2. 2 Water’s Importance to Life • Life on Earth began in water and evolved there for 3 billion years • The abundance of water is a major reason Earth is habitable – Modern life still remains tied to water – Your cells are composed of 70%– 95% water • Water has unique properties that make it a lifesupporting substance. • Properties stem from structure of molecule
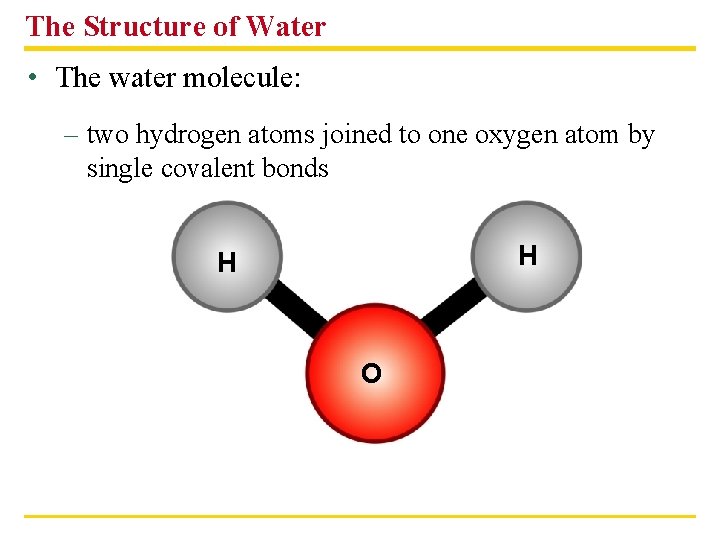
The Structure of Water • The water molecule: – two hydrogen atoms joined to one oxygen atom by single covalent bonds H H O
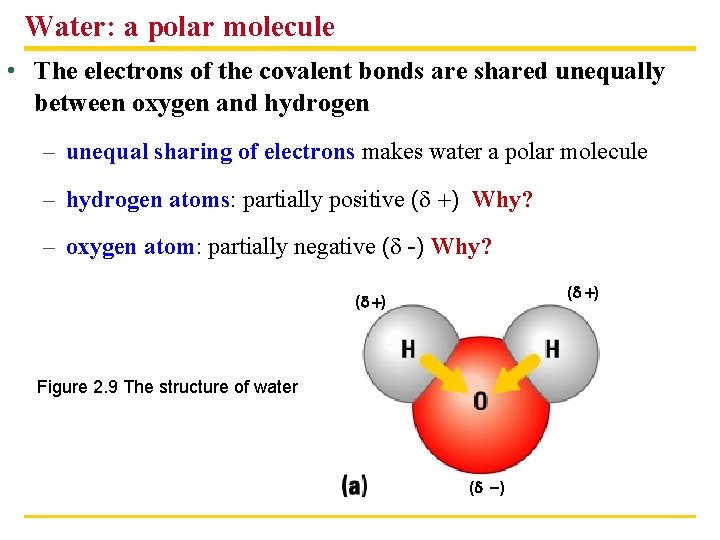
Water: a polar molecule • The electrons of the covalent bonds are shared unequally between oxygen and hydrogen – unequal sharing of electrons makes water a polar molecule – hydrogen atoms: partially positive (d ) Why? – oxygen atom: partially negative (d -) Why? (d ) Figure 2. 9 The structure of water (d )
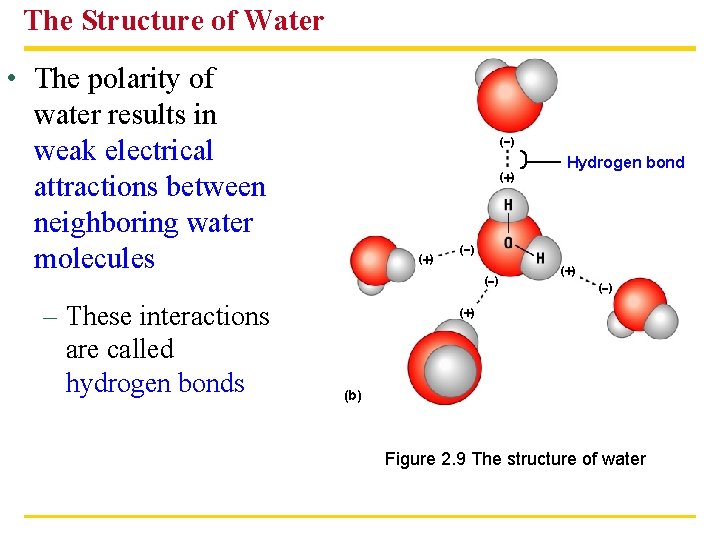
The Structure of Water • The polarity of water results in weak electrical attractions between neighboring water molecules – These interactions are called hydrogen bonds ( ) ( ) Hydrogen bond ( ) ( ) ( ) (b) Figure 2. 9 The structure of water

Water’s Life-Supporting Properties • The polarity of water molecules and the hydrogen bonding that results explain most of water’s lifesupporting properties – Cohesion and adhesion – High surface tension – High heat capacity – High heat of vaporization – Varying density
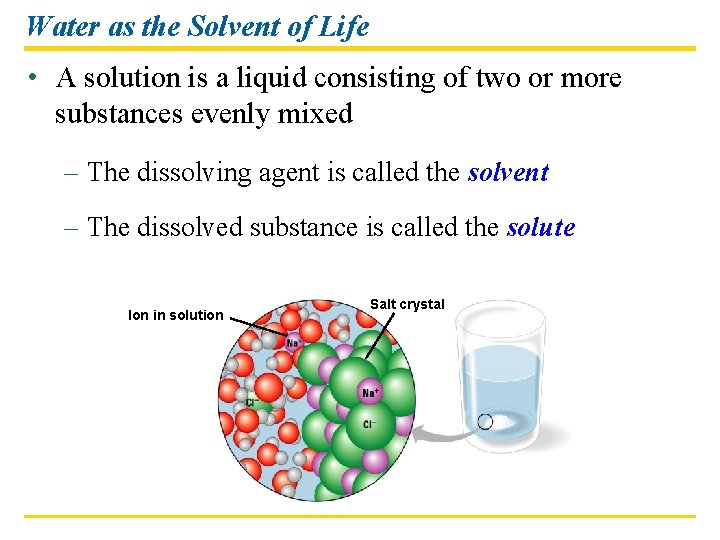
Water as the Solvent of Life • A solution is a liquid consisting of two or more substances evenly mixed – The dissolving agent is called the solvent – The dissolved substance is called the solute Ion in solution Salt crystal
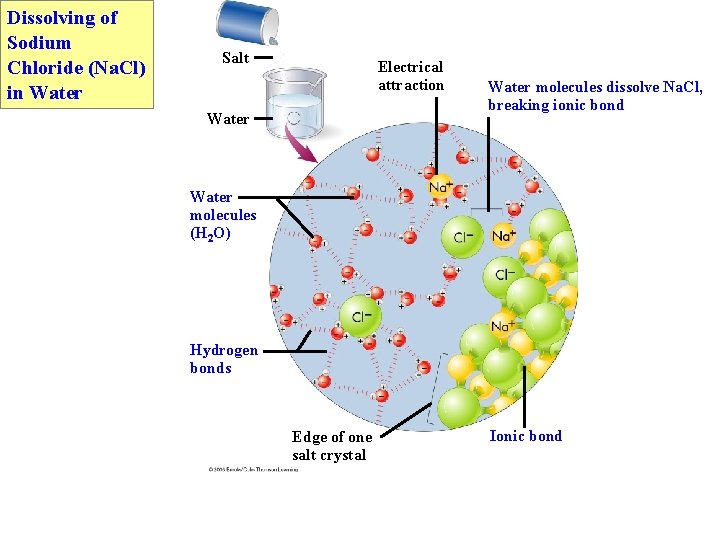
Dissolving of Sodium Chloride (Na. Cl) in Water Salt Electrical attraction Water molecules dissolve Na. Cl, breaking ionic bond Water molecules (H 2 O) Hydrogen bonds Edge of one salt crystal Ionic bond

The Cohesion of Water • Water molecules stick together as a result of hydrogen bonding – This is called cohesion – Cohesion is vital for water transport in plants Microscopic tubes

• Surface tension – is the measure of how difficult it is to stretch or break the surface of a liquid – Hydrogen bonds give water an unusually high surface tension Figure 2. 13

Water Moderates Temperature • Because of hydrogen bonding, water has a strong resistance to temperature change • Water can absorb and store large amounts of heat while only changing a few degrees in temperature – Earth’s Oceans cause temperatures to stay within limits that permit life
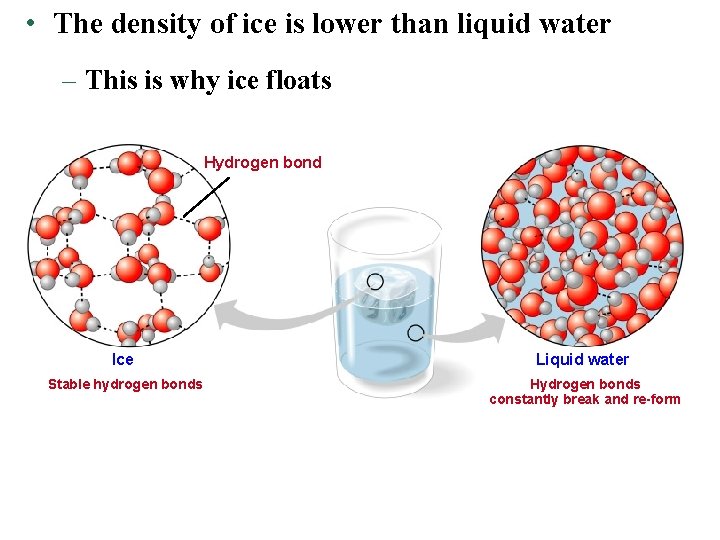
• The density of ice is lower than liquid water – This is why ice floats Hydrogen bond Ice Liquid water Stable hydrogen bonds Hydrogen bonds constantly break and re-form

• Water is a solvent. § Due to polarity and H-bonding, water dissolves many substances. § Hydrophilic – molecules attracted to water § Hydrophobic – molecules not attracted to water § Water causes Na. Cl to dissociate. H O H+ – + + O– H H Na+ Cl– The salt Na. Cl dissociates in water. +

• Cohesion § Ability of water molecules to cling to each other due to hydrogen bonding • Adhesion § Ability of water molecules to cling to other polar surfaces • Allows water to be excellent transport system in and outside of living organisms. • Contributes to water transport in plants
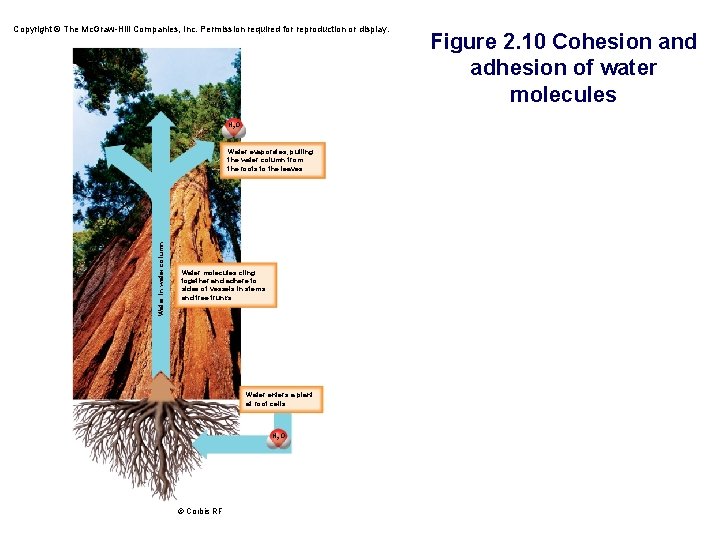
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H 2 O Water in water column Water evaporates, pulling the water column from the roots to the leaves. Water molecules cling together and adhere to sides of vessels in stems and tree trunks. Water enters a plant at root cells. H 2 O © Corbis RF Figure 2. 10 Cohesion and adhesion of water molecules

• Water has a high surface tension. § Water molecules at the surface cling more tightly to each other than to the air above. § Mainly due to hydrogen bonding
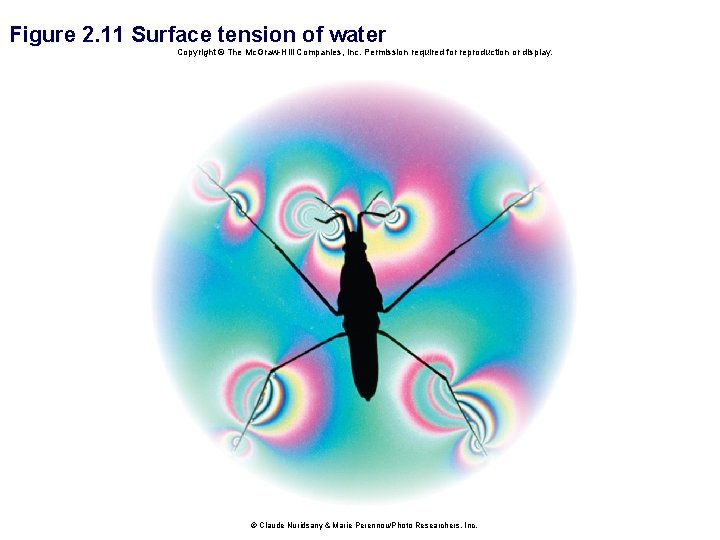
Figure 2. 11 Surface tension of water Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. © Claude Nuridsany & Marie Perennou/Photo Researchers, Inc.

• High heat capacity § The many hydrogen bonds linking water molecules allow water to absorb heat without greatly changing its temperature. § Temperature of water rises and falls slowly. • High heat of vaporization § Takes a great deal of energy to break H bonds for evaporation § Heat is dispelled as water evaporates.

Figure 2. 12 Heat of vaporization Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. a. b. a: © The Mc. Graw-Hill Companies, Inc. /Jill Braaten, photographer; b: © Super. Stock RF

• Water is less dense than ice. § Unlike other substances, water expands as it freezes. § Ice floats rather than sinks. § It makes life possible in water. § Ice acts as an insulator.
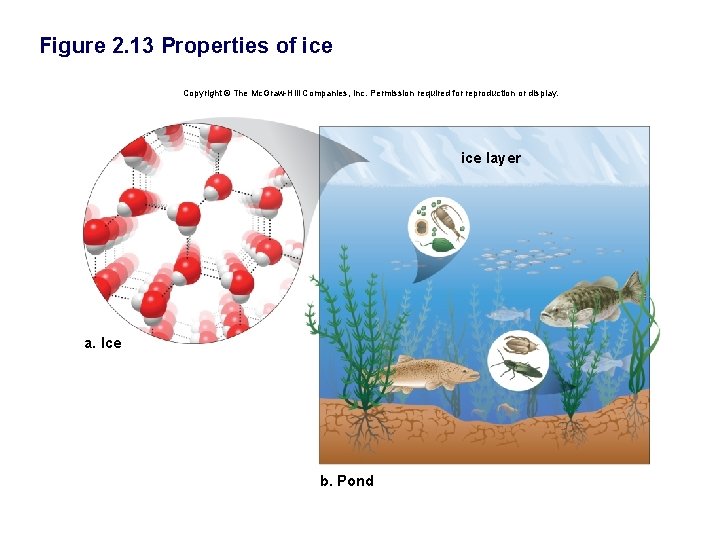
Figure 2. 13 Properties of ice Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. ice layer a. Ice b. Pond

The Biological Significance of Ice Floating • When water molecules get cold, they move apart, forming ice – A chunk of ice has fewer molecules than an equal volume of liquid water • Since ice floats, ponds, lakes, and even the oceans do not freeze solid – Marine life could not survive if bodies of water froze solid
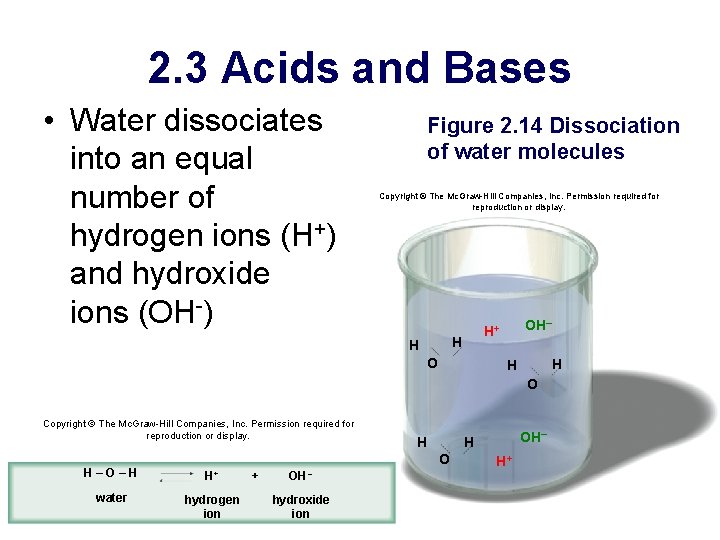
2. 3 Acids and Bases • Water dissociates into an equal number of hydrogen ions (H+) and hydroxide ions (OH-) Figure 2. 14 Dissociation of water molecules Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H H OH– H+ O H H O Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H O H–O–H H+ water hydrogen ion + OH – hydroxide ion OH– H H+
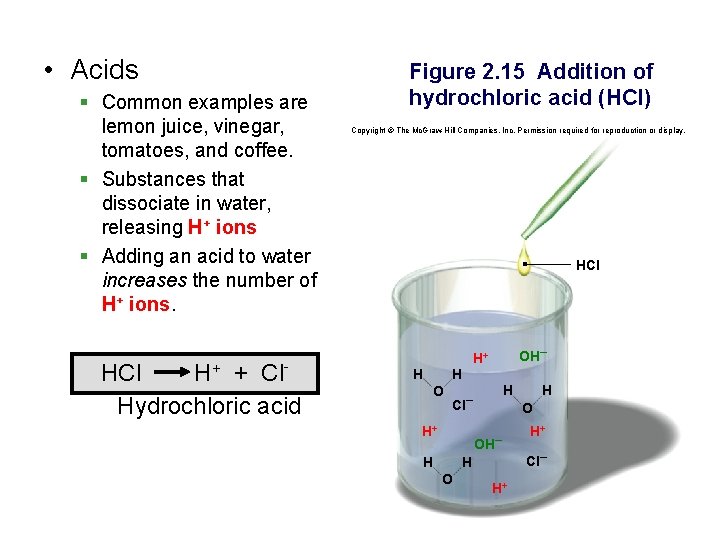
• Acids § Common examples are lemon juice, vinegar, tomatoes, and coffee. § Substances that dissociate in water, releasing H+ ions § Adding an acid to water increases the number of H+ ions. HCl H+ + Cl. Hydrochloric acid Figure 2. 15 Addition of hydrochloric acid (HCl) Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. HCl OH– H+ H H O H Cl– H+ O OH– H H O H H+ H+ Cl–
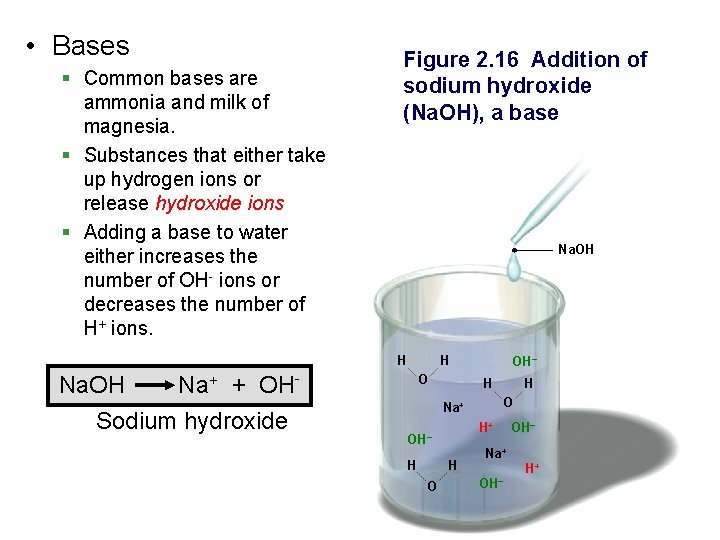
• Bases § Common bases are ammonia and milk of magnesia. § Substances that either take up hydrogen ions or release hydroxide ions § Adding a base to water either increases the number of OH- ions or decreases the number of H+ ions. Figure 2. 16 Addition of sodium hydroxide (Na. OH), a base Na. OH H Na. OH Na+ + OHSodium hydroxide H O OH– H O Na+ H+ OH– H H O H Na+ OH– H+
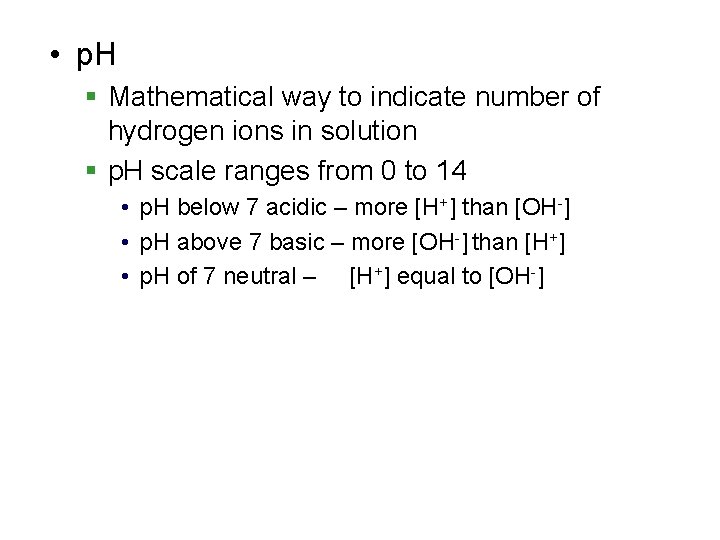
• p. H § Mathematical way to indicate number of hydrogen ions in solution § p. H scale ranges from 0 to 14 • p. H below 7 acidic – more [H+] than [OH-] • p. H above 7 basic – more [OH-] than [H+] • p. H of 7 neutral – [H+] equal to [OH-]

Acids, Bases, and p. H • Acid § A chemical compound that donates H+ ions to solutions § Taste sour • Base § A compound that …. • accepts H+ ions and removes them from solution • Dissolves in water to produce hydroxide ions, OH • Taste bitter
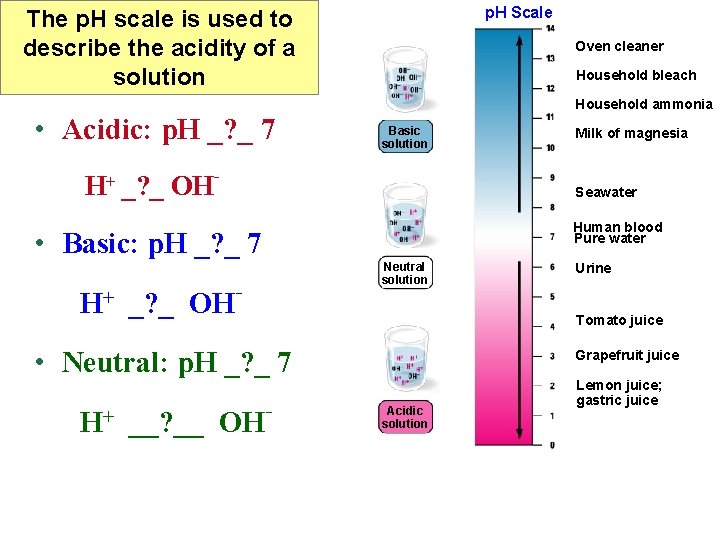
p. H Scale The p. H scale is used to describe the acidity of a solution • Acidic: p. H _? _ 7 Oven cleaner Household bleach Household ammonia Basic solution H+ _? _ OH- Seawater Human blood Pure water • Basic: p. H _? _ 7 H+ _? _ OH Neutral solution - __? __ OH Urine Tomato juice • Neutral: p. H _? _ 7 H+ Milk of magnesia - Grapefruit juice Acidic solution Lemon juice; gastric juice
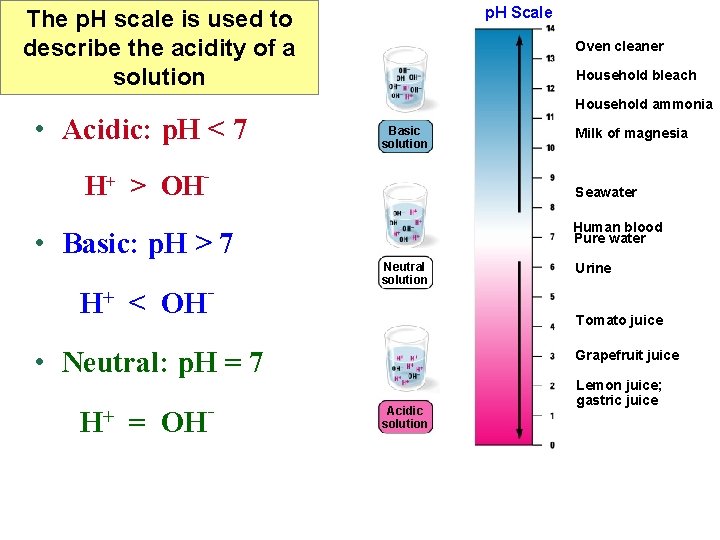
p. H Scale The p. H scale is used to describe the acidity of a solution • Acidic: p. H < 7 Oven cleaner Household bleach Household ammonia Basic solution H+ > OH- Seawater Human blood Pure water • Basic: p. H > 7 H+ < OH - Neutral solution = OH Urine Tomato juice • Neutral: p. H = 7 H+ Milk of magnesia - Grapefruit juice Acidic solution Lemon juice; gastric juice

• Buffer § Chemical or combination of chemicals that keeps p. H within normal limits § Resist p. H change by taking up excess H+ or OH§ p. H of blood is about 7. 4 – maintained by buffer
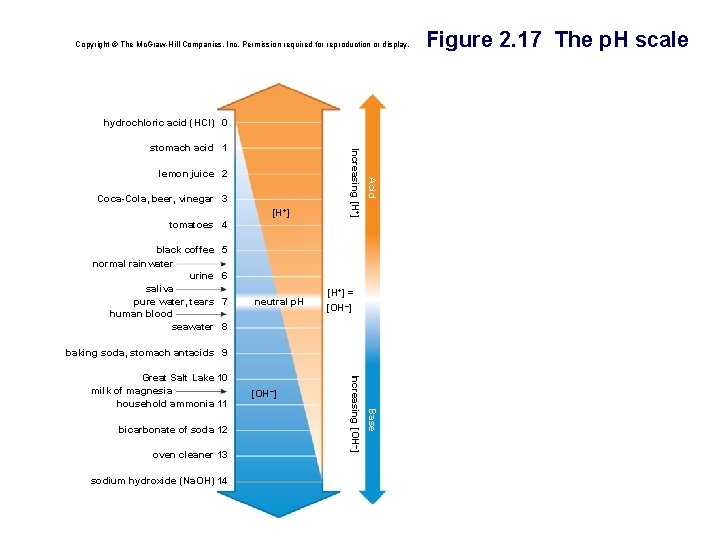
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. hydrochloric acid (HCI) 0 Coca-Cola, beer, vinegar 3 [H+] Acid lemon juice 2 Increasing [H+] stomach acid 1 tomatoes 4 black coffee normal rainwater urine saliva pure water, tears human blood seawater 5 6 7 neutral p. H [H+] = [OH–] 8 baking soda, stomach antacids 9 oven cleaner 13 sodium hydroxide (Na. OH) 14 Base bicarbonate of soda 12 [OH–] Increasing [OH–] Great Salt Lake 10 milk of magnesia household ammonia 11 Figure 2. 17 The p. H scale
Self-test/Review Questions Use these questions as a self test and then discuss your responses with your study group/classmates—your responses will not be collected. 1. Why is carbon dioxide gas, CO 2, classified as a compound but nitrogen gas, N 2, is not? 2. Which of the following are compounds? Elements? : C 6 H 12 O 6, CH 4, O 2, Cl 2, HCl, Mg. Cl 2, Fe, Ca, Ne, Na. I, I 3. What is the difference between an atom and an ion? Give examples of each to support your response. 4. Which subatomic particle determines the identity of an atom? 5. Which subatomic particle determines the chemical properties of an atom?

Self-test/Review Questions 6. A carbon atom has 6 protons, and the most common isotope of carbon has 6 neutrons. A radioactive isotope of carbon has 8 neutrons. What are the atomic numbers and the mass numbers of the stable and radioactive forms of carbon? 7. Explain the difference between an ionic and covalent bond in terms of what happens to the electrons in the outer shell of the participating atoms. 8. Sodium fluoride, Na. F, is often added to toothpaste to both kill bacteria that cause cavities. It also helps to harden the enamel of teeth thus helping it resist cavities. Is sodium fluoride an ionic or covalent compound? How do you know? Explain your reasoning. 9. Is carbon dioxide an ionic or covalent compound? How do you know? Explain your reasoning.
Self-test/Review Questions (cont. ) 10. Why are the following incorrect structures for the substances below? Rewrite their structures with the correct number of chemical bonds. a. Carbon dioxide gas: O—C—O b. Oxygen gas: O—O c. Nitrogen gas: N—N 11. Explain how water’s versatility as a solvent results from the fact that water is polar molecule. 12. A bottle of Pepsi consists mostly of sugar dissolved in water, with some carbon dioxide gas that makes fizzy and makes the p. H less than 7. Describe Pepsi using the following terms: solute, solvent, acidic, aqueous solution

Self-test/Review Questions (cont. ) 13. Which of the following are chemical changes? Physical changes? If possible, write the balanced chemical equation for those that are a chemical change. a. The alcoholic fermentation in Yeast in which yeast produce ethanol, C 2 H 5 OH, and carbon dioxide, CO 2, from the sugar glucose, C 6 H 12 O 6 b. Water boils to form steam c. The healing of a cut finger d. Cutting a piece of wood with a saw e. Potassium metal, K, and chlorine gas (Cl 2) combine to form potassium chloride. f. The rusting of iron, Fe, to produce rust, iron (III) oxide (Fe 2 O 3)
Self-test/Review Questions (cont. ) 14. Which of these is not a subatomic particle? a) proton; b) ion; c) neutron; d) electron 15. The outermost electron shell of every Noble Gas element (except Helium) has ___ electrons. a) 1; b) 2; c) 4; d) 6; e) 8 16. An organic molecule is likely to contain all of these elements except ___. a) C; b) H; c) O; d) Ne; e) N 17. The chemical bond between water molecules is a ___ bond. a) ionic; b) polar covalent; c) nonpolar covalent; d) hydrogen 18. A solution with a p. H of 7 has ___ times more H ions than a solution of p. H 9. a) 2; b) 100; c) 1000; d) 9; e) 90 19. The type of chemical bond formed when electrons are shared between atoms is a ___ bond. a) ionic; b) covalent; c) hydrogen
Self-test/Review Questions (cont. ) 20. The type of chemical bond formed when oppositely charged particles are attached to each other is a ___ bond. a) ionic; b) covalent; c) hydrogen 21. Carbon has an atomic number of 6. This means it has ___. a) six protons; b) six neutrons; c) six protons plus six neutrons; d) six neutrons and six electrons 22. Each of the isotopes of hydrogen has ___ proton(s). a) 3; b) 1; c) 2; d) 92; e) 1/2 23. A molecule is ___. a) a mixture of various components that can vary; b) a combination of many atoms that will have different ratios; c) a combination of one or more atoms that will have a fixed ratio of its components; d) more important in a chemistry class than in a biology class
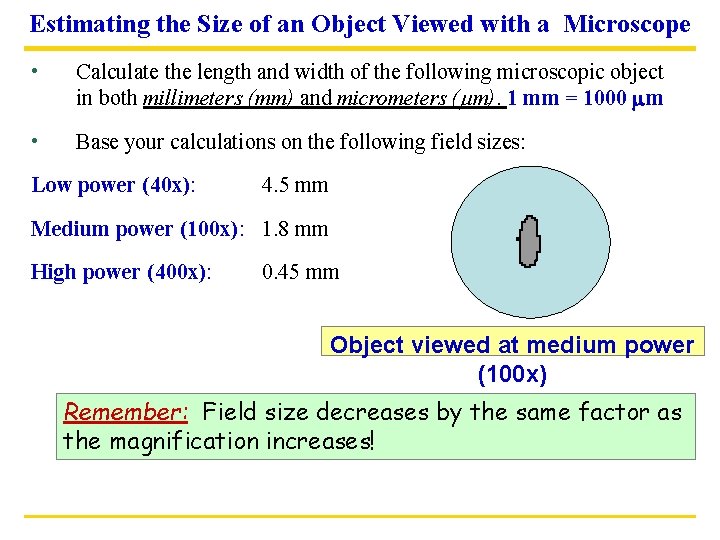
Estimating the Size of an Object Viewed with a Microscope • Calculate the length and width of the following microscopic object in both millimeters (mm) and micrometers (mm). 1 mm = 1000 mm • Base your calculations on the following field sizes: Low power (40 x): 4. 5 mm Medium power (100 x): 1. 8 mm High power (400 x): 0. 45 mm Object viewed at medium power (100 x) Remember: Field size decreases by the same factor as the magnification increases!
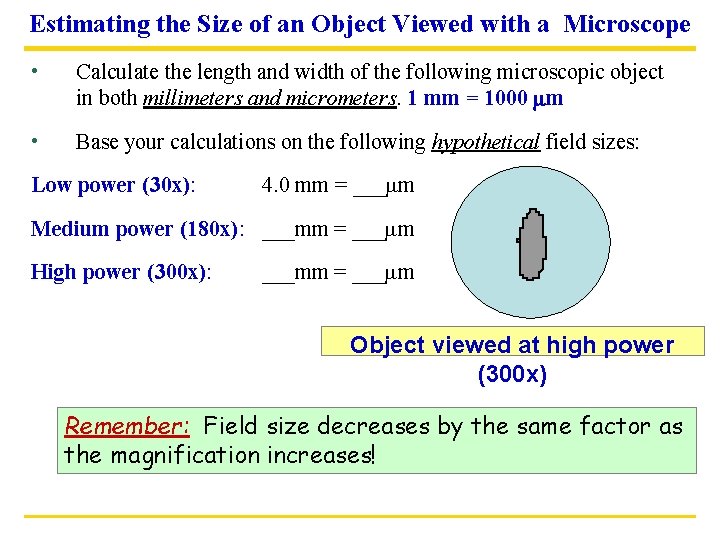
Estimating the Size of an Object Viewed with a Microscope • Calculate the length and width of the following microscopic object in both millimeters and micrometers. 1 mm = 1000 mm • Base your calculations on the following hypothetical field sizes: Low power (30 x): 4. 0 mm = ___mm Medium power (180 x): ___mm = ___mm High power (300 x): ___mm = ___mm Object viewed at high power (300 x) Remember: Field size decreases by the same factor as the magnification increases!
- Slides: 66