Essential Questions What is the definition of matter

























- Slides: 33

Essential Questions Ø What is the definition of matter? Ø Differentiate between physical and chemical properties. Ø Illustrate the states of matter Ø Know the changes of states of matter Ø What are the signs that tell you that a chemical reaction might be taking place?

Classification of Matter? Does it Matter? What’s the Matter?

So, What is Matter ? Ø Matter is defined as anything that has : Mass (inertia a resistance to change in movement) Takes up space (volume)

Physical Property Ø Any quality or condition of a substance that can be observed or measured without changing the substances identity. Examples: color, solubility, odor, hardness, melting point, and state

Physical States of Matter There’s 4 of them ! Ø SOLIDS Have definite (or fixed) shape and volume The particles in a solid are held fairly rigidly in place.

Physical States of Matter There’s 4 of them ! Ø LIQUIDS Have a definite volume but no fixed shape. The particles in a liquid are free to flow around each other

Physical States of Matter There’s 4 of them ! Ø GASES Have neither definite or fixed shape or volume. The particles in a gas are: widely disbursed, interact weakly, move independently at high speed, and completely fill any container they occupy.

4 th Type of Matter PLASMA Gases whose particles are so hot they have acquired an electrical charge.

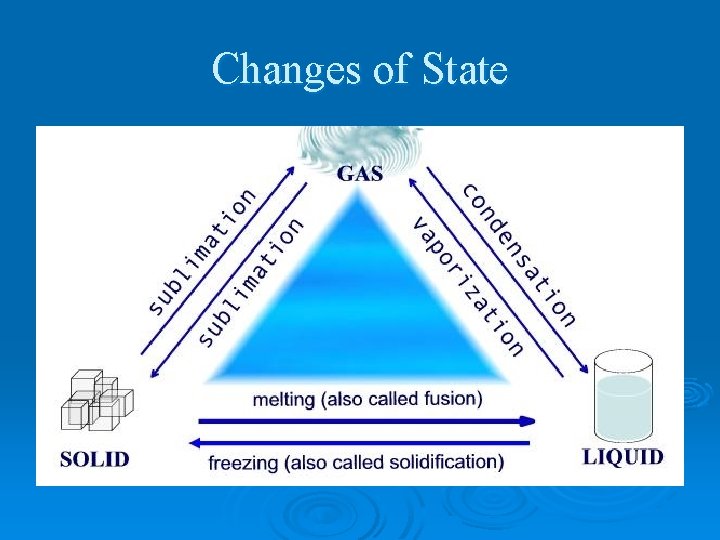
Changes of State

Physical Changes in Matter Ø change in a substance that doesn’t change the identity of the substance Ø Ex. grinding, cutting, melting, boiling Ø Includes all changes of state (physical changes of a substance from one state to another)

Chemical Changes in Matter Ø a change in which a substance is converted into a different substance Ø same as chemical reaction Ø doesn’t change the amount of matter present Ø reactants – substances that react Ø products – substances that form

Signs of Chemical Change Ø Energy is always absorbed or given off Ø Change in color or odor Ø Production of a gas Ø Irreversibility

Chemical or Physical? Ø Cookies are baked Ø Water boils Ø Salt dissolves in water Ø Milk spoils Ø A metal chair rusts Ø Paper is torn Ø A tree burns down

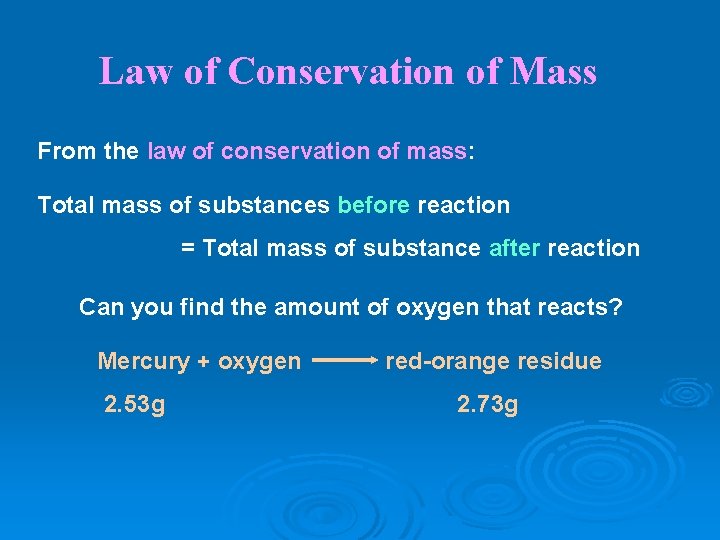
Law of Conservation of Mass Ø In any physical change or chemical reaction, mass is neither created nor destroyed; it is conserved.

Law of Conservation of Mass From the law of conservation of mass: Total mass of substances before reaction = Total mass of substance after reaction Can you find the amount of oxygen that reacts? Mercury + oxygen 2. 53 g red-orange residue 2. 73 g

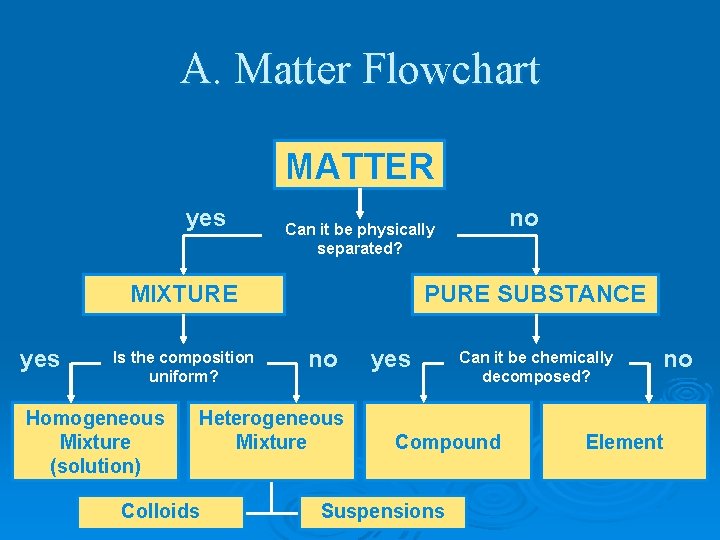
EQ’s Ø How is matter classified? Ø What are pure substances? Ø What are mixtures? Ø What are homogeneous mixtures? Ø What are heterogeneous mixtures?

A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element

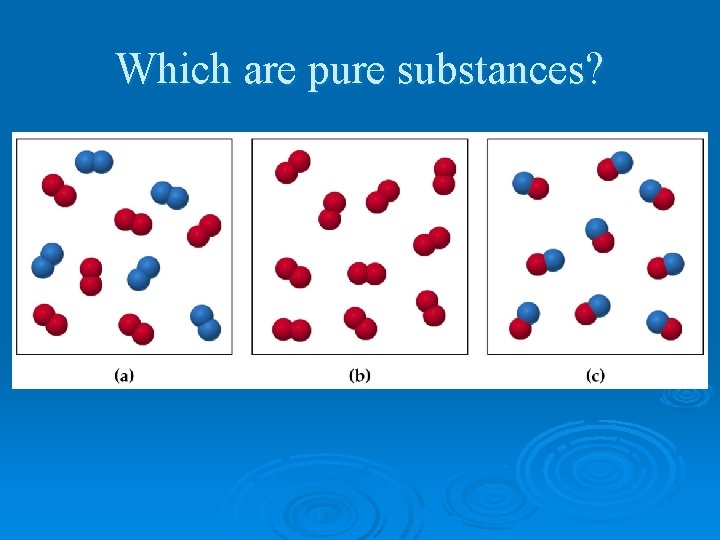
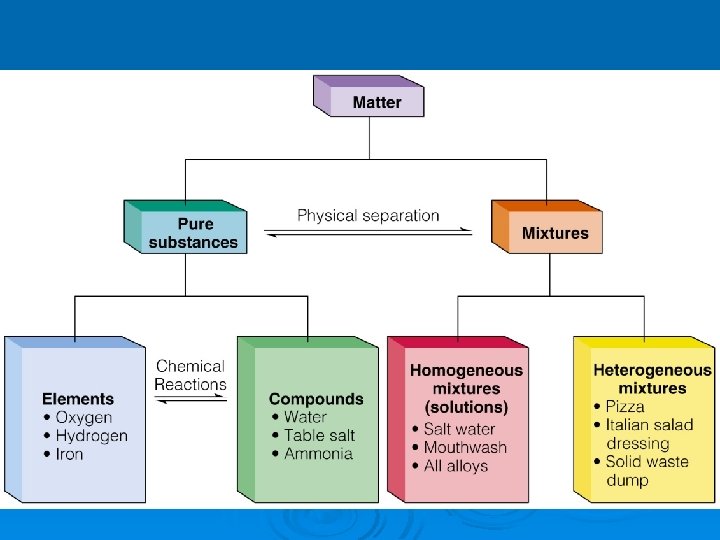
Pure Substances Ø every sample has same: l l characteristic properties composition Ø are made of: l one type of atom: element • Ex: iron, gold, oxygen l 2 or more types of atoms: compound • Ex: salt, sugar, water

Which are pure substances?

Mixtures Ø blend of 2 or more types of matter Ø each component keeps its own identity and properties Ø the components are only physically mixed Ø can be separated using physical means Ø properties of the mixture a combination of the componenent’s properties

Homogeneous Mixtures Ø Ø Ø also called solution uniform in composition no visible parts Ex: Ø vinegar Ø clear air Ø salt water Ø brass

Mixtures

Heterogeneous Mixtures not uniform in composition Ø visible parts Ø Ex: Ø soil Ø concrete Ø blood Ø chocolate chip cookies Ø sand in water Ø iced tea with ice

Substance or Mixture? Ø A homogeneous mixture looks like a substance Ø Is the material in question always a single kind of material? For instance, there are different grades of gasoline and different kinds of cough syrup Ø Is the material in question physically separable?

EQ’s Ø What are five physical separation techniques?

Physical Separation Techniques Filtration- solid part is trapped by filter paper and the liquid part runs through the paper Ø Vaporization- where the liquid portion is evaporated off to leave solid Ø

Physical Separation Techniques Decanting- when liquid is poured off after solid has settled to bottom Ø Centrifuge- machine that spins a sample very quickly so that components with different densities will separate Ø

Physical Separation Techniques Ø Paper Chromatography- used to separate mixtures because different parts move quicker on paper than other

Classify the following as a substance or a mixture Ø Silver Ø Alphabet soup Ø Salt water Ø Table salt (sodium chloride) Ø Motor oil


Practice Determine whether each of the following is element, compound, homogeneous mixture or heterogeneous mixture. Ø Ø Ø Ø Ø air zinc chlorine granite aluminum sugar in water blood sucrose stainless steel sodium chloride Ø Ø Ø Ø Ø brass whole milk apple table salt soft drinks vinegar concrete sodium baking soda (Na. HCO 3) gravel

Ø How do you separate different substances from a mixture? You have just been given the latest creation of your science teacher. The teacher presents you with a mixture of sawdust, iron filings, salt, and sand. He needs to separate the mixture and wants your help. You are to separate the mixture and find the total mass of each of the substances in the mixture! You are to describe your proposed separation plan. Present your plan to your teacher for approval. Ø You will then conduct the experiment and separate the mixture Ø Upon completion of the experiment each member of the group will submit a final paper Ø

The Final Paper Must Include the Following: *Introduction: Define a mixture and properties of the ingredients in this mixture *Materials: List of lab equipment used *Procedures: Numbered step by step procedures to took to separate the mixture *Results: Mass of each substance *Conclusion: A description of problems you encountered while conducting the lab Ideas as to how you could improve your methods to better separate each ingredient.