Essential Organic Chemistry Paula Yurkanis Bruice Chapter 4

Essential Organic Chemistry Paula Yurkanis Bruice Chapter 4 Alkenes: Structure, Nomenclature, Stability, and an Introduction to Reactivity William Setzer, Bernhard Vogler, Mary Setzer University of Alabama - Huntsville Copyright © 2010 Pearson Education, Inc.
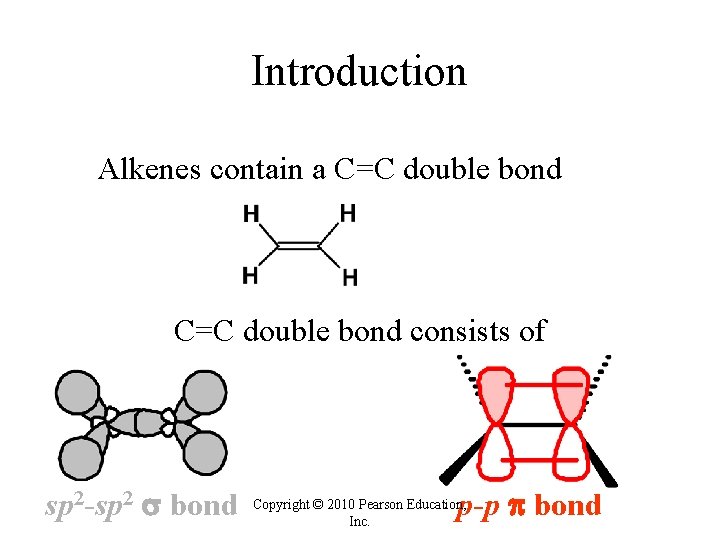
Introduction Alkenes contain a C=C double bond consists of sp 2 -sp 2 bond p-p bond Copyright © 2010 Pearson Education, Inc.
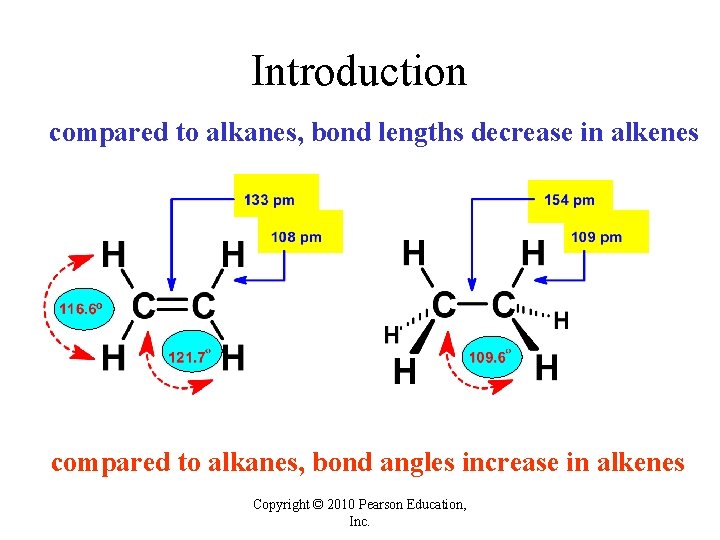
Introduction compared to alkanes, bond lengths decrease in alkenes compared to alkanes, bond angles increase in alkenes Copyright © 2010 Pearson Education, Inc.
Introduction Ø Typical representatives are • Ethene, plant growth hormone Copyright © 2010 Pearson Education, Inc.
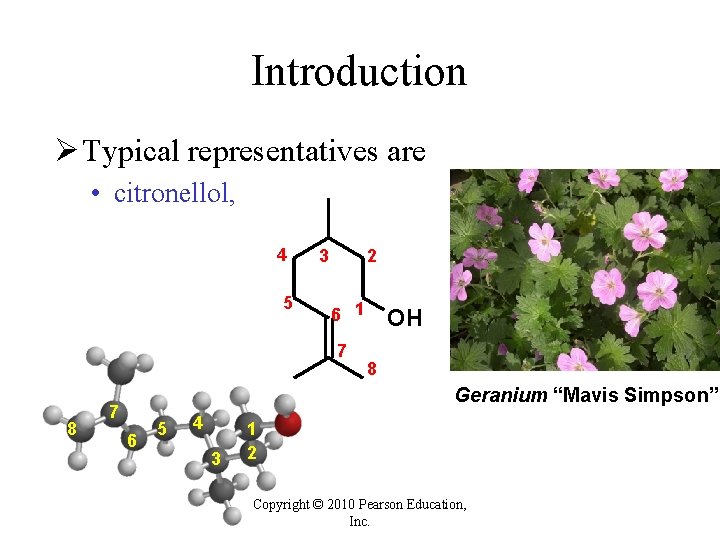
Introduction Ø Typical representatives are • citronellol, 4 5 3 2 6 1 7 8 OH 8 Geranium “Mavis Simpson” 7 6 5 4 3 1 2 Copyright © 2010 Pearson Education, Inc.
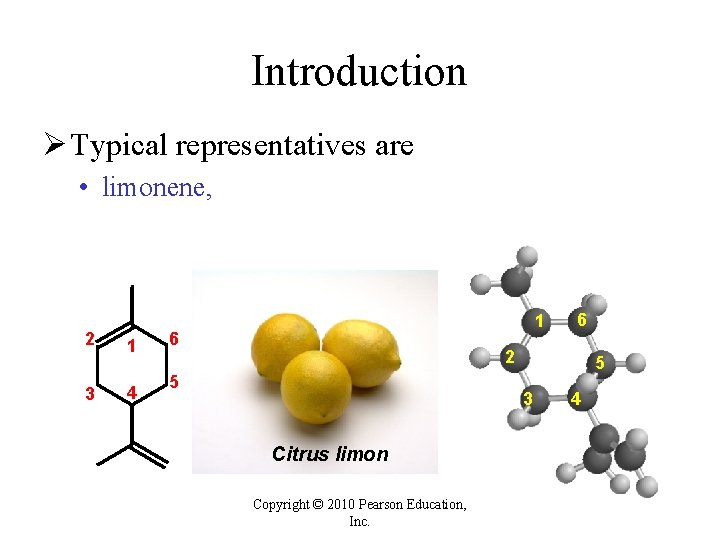
Introduction Ø Typical representatives are • limonene, 2 3 1 4 1 6 6 2 5 5 3 Citrus limon Copyright © 2010 Pearson Education, Inc. 4
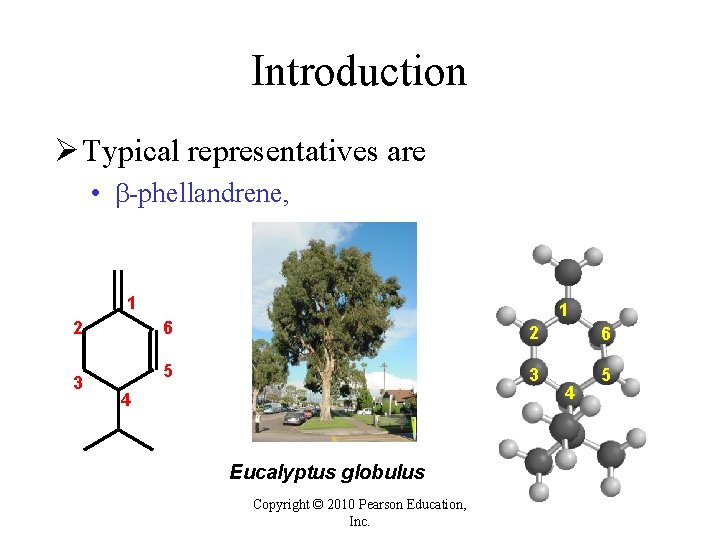
Introduction Ø Typical representatives are • -phellandrene, 1 2 3 1 6 2 6 5 3 5 4 Eucalyptus globulus Copyright © 2010 Pearson Education, Inc. 4
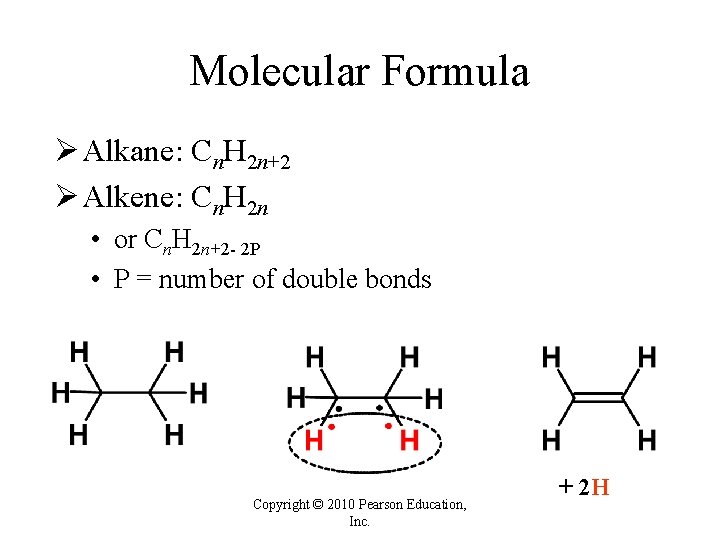
Molecular Formula Ø Alkane: Cn. H 2 n+2 Ø Alkene: Cn. H 2 n • or Cn. H 2 n+2 - 2 P • P = number of double bonds Copyright © 2010 Pearson Education, Inc. + 2 H
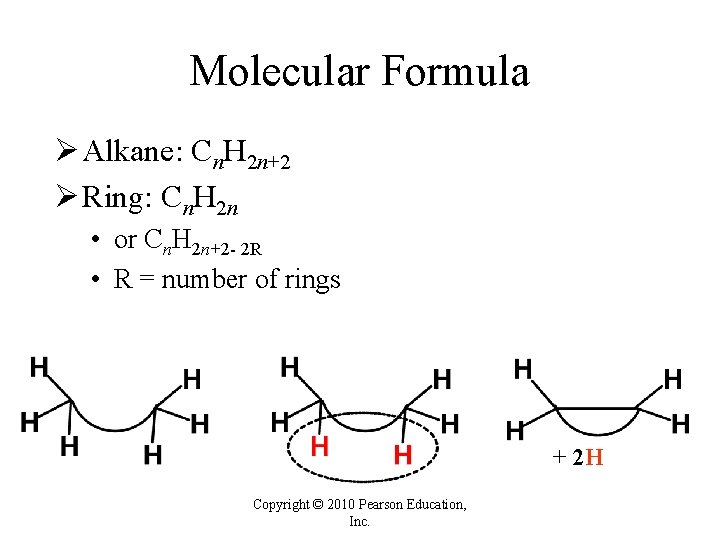
Molecular Formula Ø Alkane: Cn. H 2 n+2 Ø Ring: Cn. H 2 n • or Cn. H 2 n+2 - 2 R • R = number of rings + 2 H Copyright © 2010 Pearson Education, Inc.
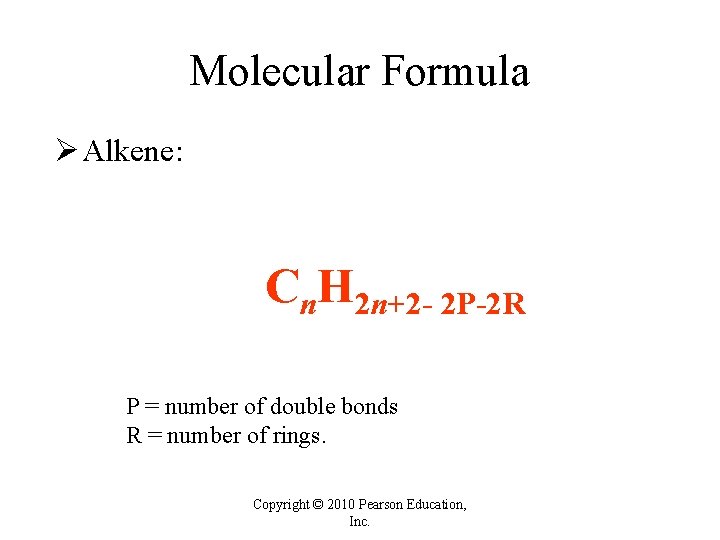
Molecular Formula Ø Alkene: Cn. H 2 n+2 - 2 P-2 R P = number of double bonds R = number of rings. Copyright © 2010 Pearson Education, Inc.

Nomenclature of Alkenes Ø Find the longest carbon chain. Ø Enumerate the carbons such that the functional group, here the double bond, gets the lowest possible number. Ø Substituents are cited before the parent longest chain, along with a number indicating its position at the chain. Copyright © 2010 Pearson Education, Inc.
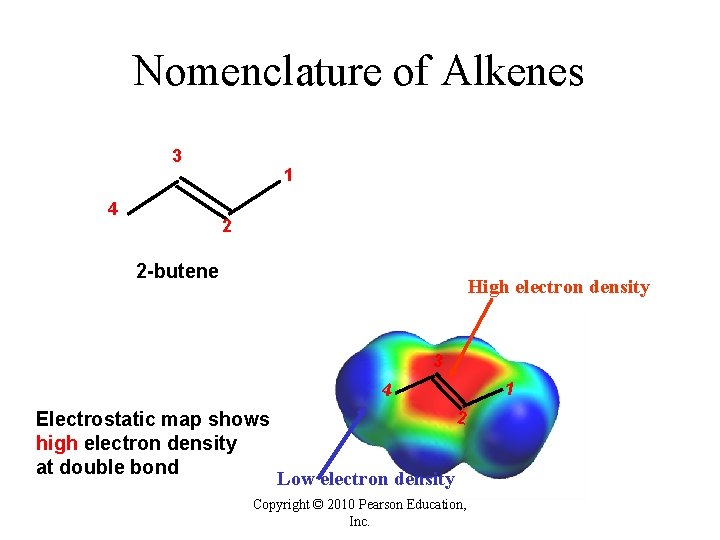
Nomenclature of Alkenes 3 4 1 2 2 -butene High electron density 3 1 4 Electrostatic map shows high electron density at double bond 2 Low electron density Copyright © 2010 Pearson Education, Inc.
Nomenclature of Alkenes 2 -hexene bond Copyright © 2010 Pearson Education, Inc.
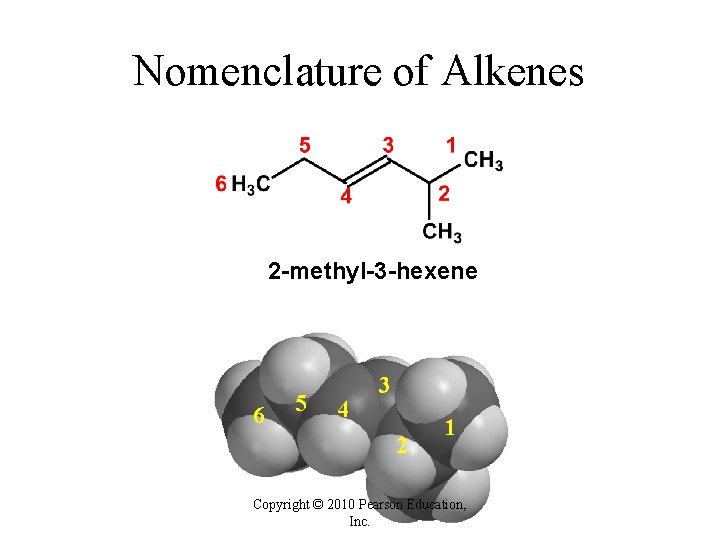
Nomenclature of Alkenes 2 -methyl-3 -hexene 6 5 4 3 2 1 Copyright © 2010 Pearson Education, Inc.
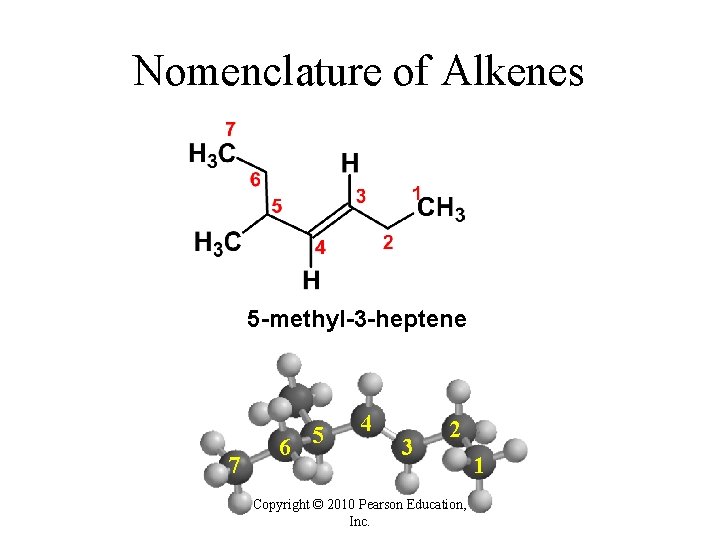
Nomenclature of Alkenes 5 -methyl-3 -heptene 7 6 5 4 3 2 Copyright © 2010 Pearson Education, Inc. 1
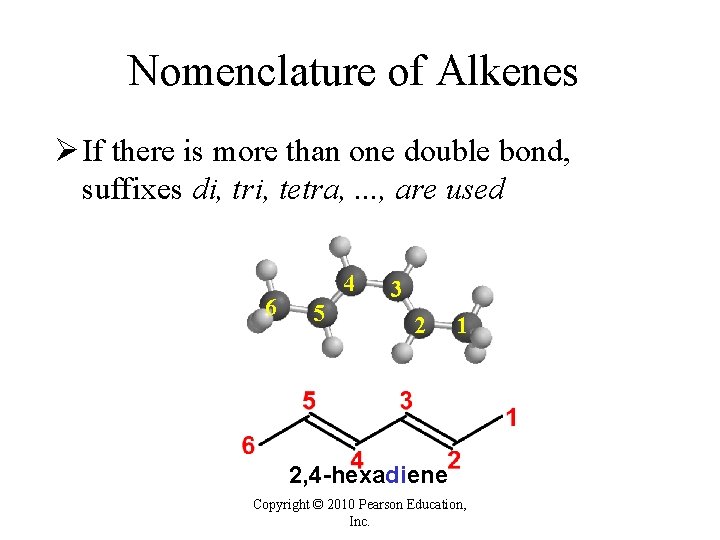
Nomenclature of Alkenes Ø If there is more than one double bond, suffixes di, tri, tetra, . . . , are used 6 4 5 3 2 1 2, 4 -hexadiene Copyright © 2010 Pearson Education, Inc.
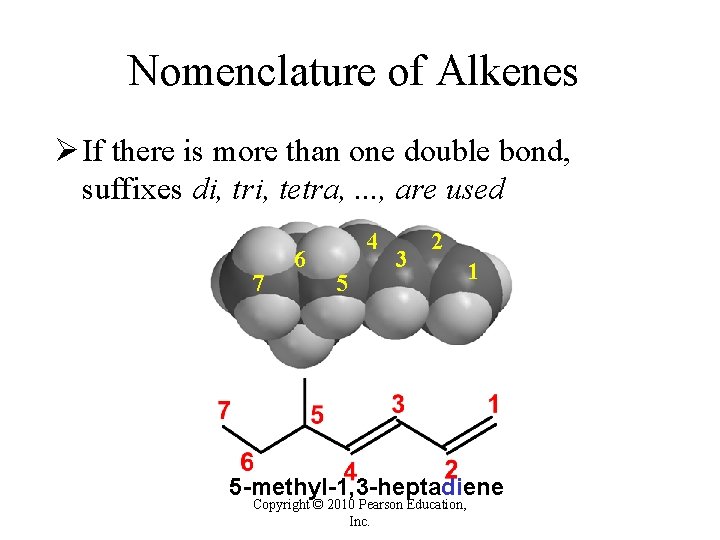
Nomenclature of Alkenes Ø If there is more than one double bond, suffixes di, tri, tetra, . . . , are used 7 6 4 5 3 2 1 5 -methyl-1, 3 -heptadiene Copyright © 2010 Pearson Education, Inc.
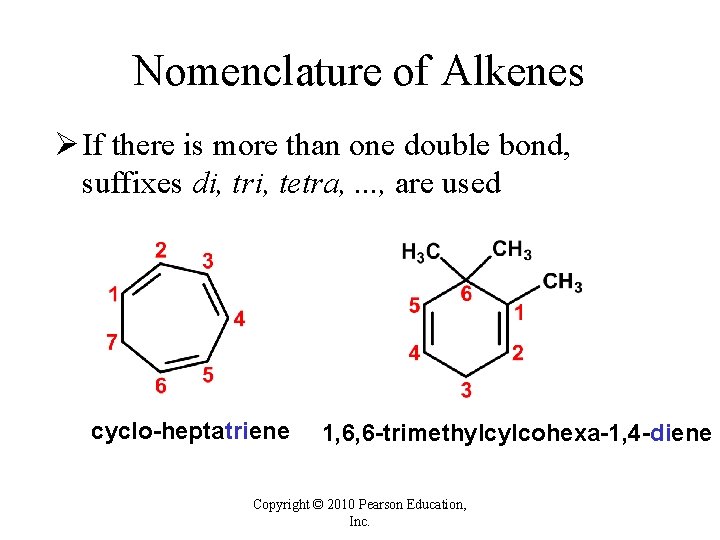
Nomenclature of Alkenes Ø If there is more than one double bond, suffixes di, tri, tetra, . . . , are used cyclo-heptatriene 1, 6, 6 -trimethylcylcohexa-1, 4 -diene Copyright © 2010 Pearson Education, Inc.
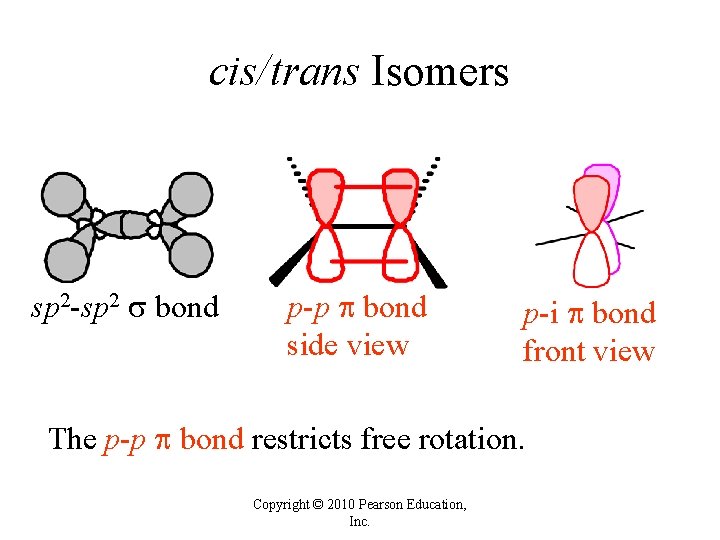
cis/trans Isomers sp 2 -sp 2 bond p-p bond side view p-i bond front view The p-p bond restricts free rotation. Copyright © 2010 Pearson Education, Inc.
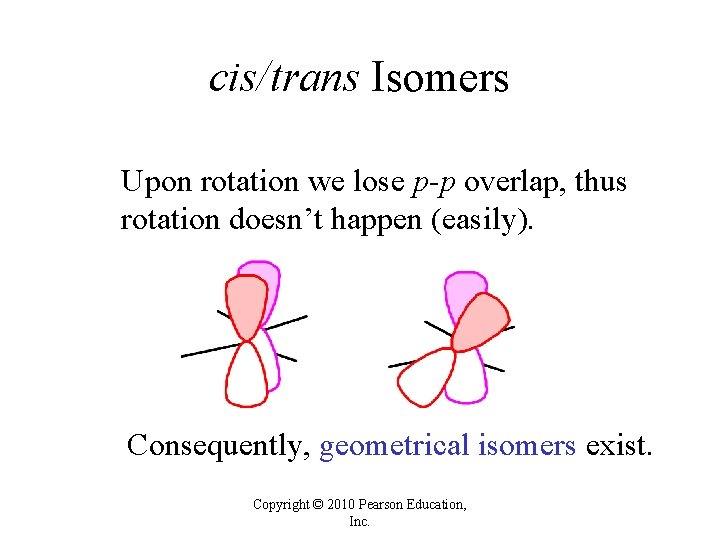
cis/trans Isomers Upon rotation we lose p-p overlap, thus rotation doesn’t happen (easily). Consequently, geometrical isomers exist. Copyright © 2010 Pearson Education, Inc.
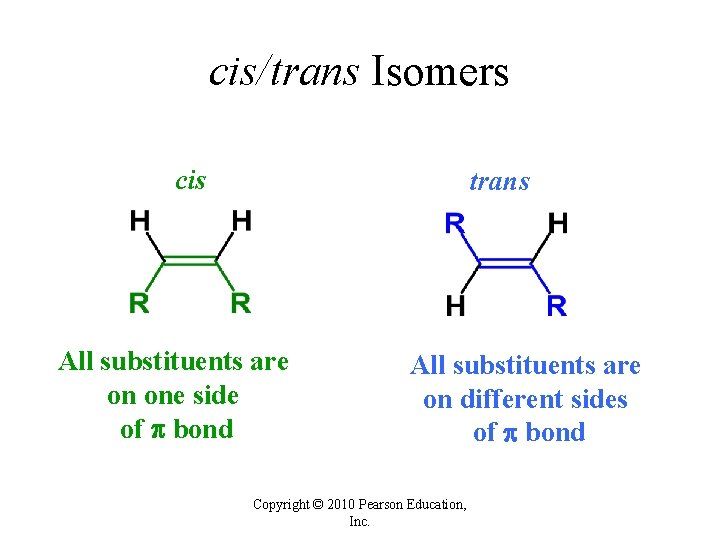
cis/trans Isomers cis trans All substituents are on one side of bond All substituents are on different sides of bond Copyright © 2010 Pearson Education, Inc.
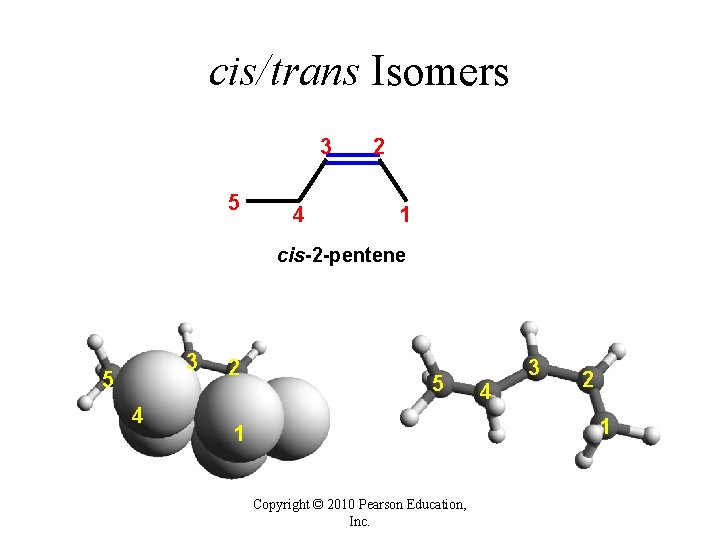
cis/trans Isomers 3 5 4 2 1 cis-2 -pentene 3 5 4 2 5 4 3 2 1 1 Copyright © 2010 Pearson Education, Inc.
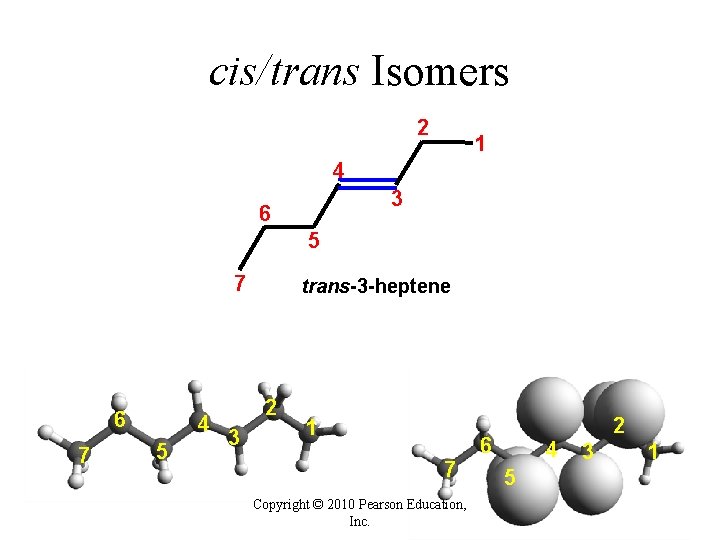
cis/trans Isomers 2 1 4 3 6 5 7 6 7 4 5 trans-3 -heptene 2 3 1 7 Copyright © 2010 Pearson Education, Inc. 6 4 5 2 3 1
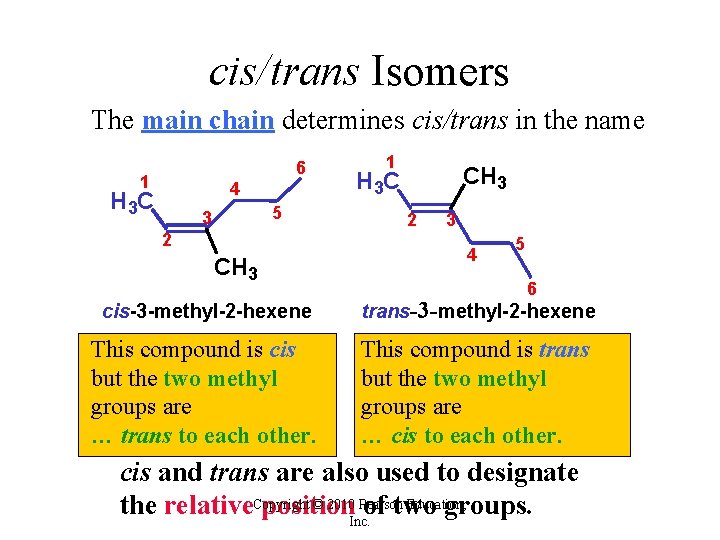
cis/trans Isomers The main chain determines cis/trans in the name 6 1 4 H 3 C 5 3 2 CH 3 1 CH 3 H 3 C 2 3 4 5 6 cis-3 -methyl-2 -hexene trans-3 -methyl-2 -hexene This compound is cis but the two methyl groups are … trans to each other. This compound is trans but the two methyl groups are … cis to each other. cis and trans are also used to designate © 2010 Pearson Education, the relative. Copyright position of two groups. Inc.
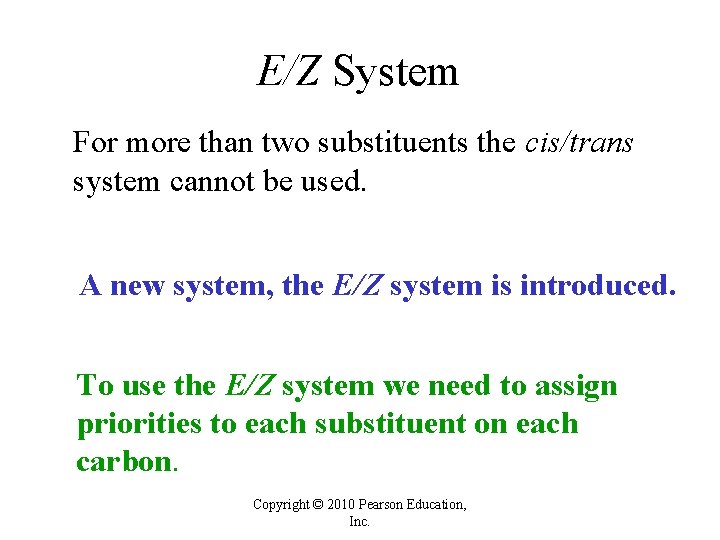
E/Z System For more than two substituents the cis/trans system cannot be used. A new system, the E/Z system is introduced. To use the E/Z system we need to assign priorities to each substituent on each carbon. Copyright © 2010 Pearson Education, Inc.
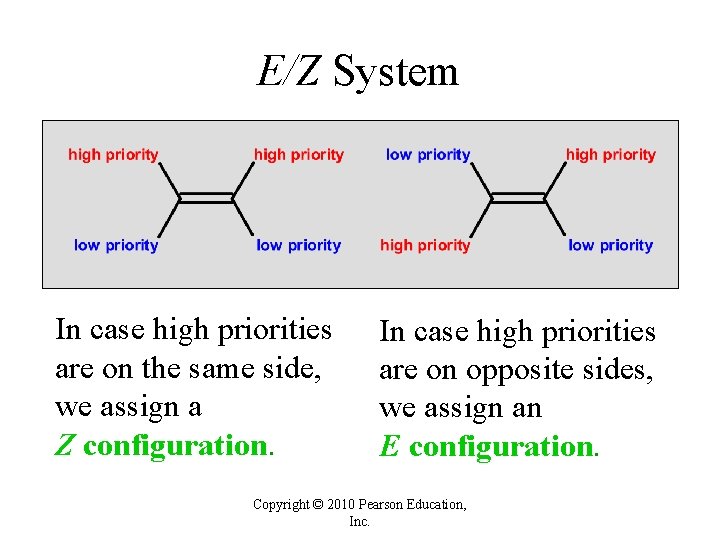
E/Z System In case high priorities are on the same side, we assign a Z configuration. In case high priorities are on opposite sides, we assign an E configuration. Copyright © 2010 Pearson Education, Inc.
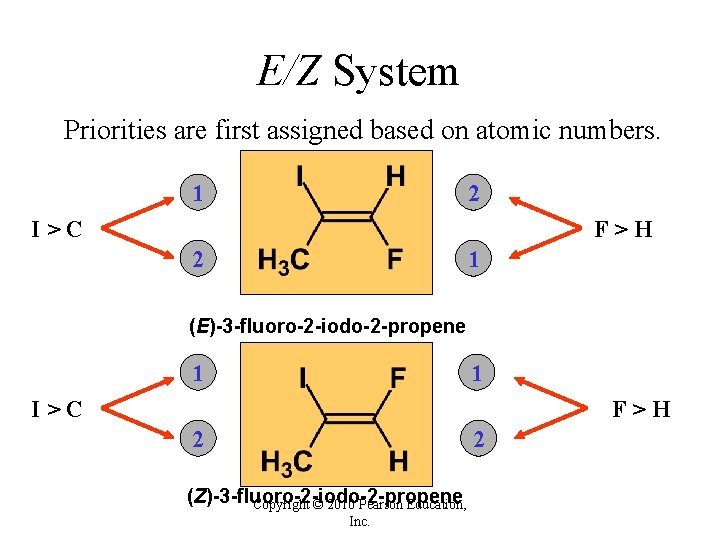
E/Z System Priorities are first assigned based on atomic numbers. 1 2 I>C F>H 2 1 (E)-3 -fluoro-2 -iodo-2 -propene 1 1 I>C F>H 2 2 (Z)-3 -fluoro-2 -iodo-2 -propene Copyright © 2010 Pearson Education, Inc.
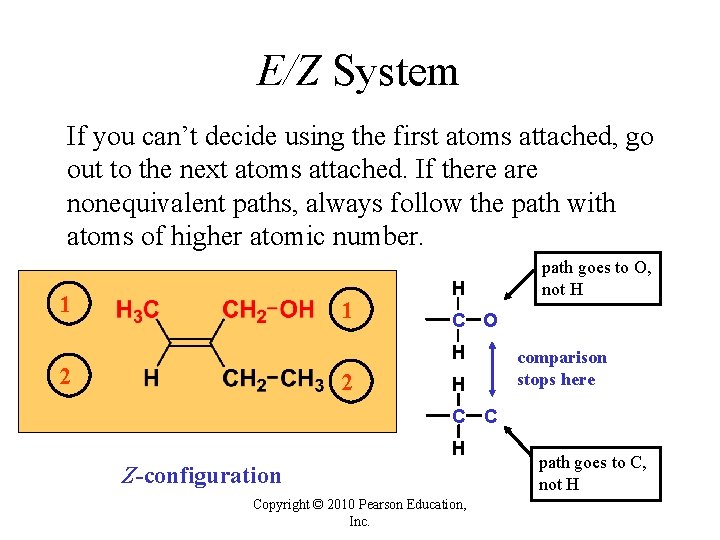
E/Z System If you can’t decide using the first atoms attached, go out to the next atoms attached. If there are nonequivalent paths, always follow the path with atoms of higher atomic number. 1 1 H C O H 2 2 path goes to O, not H H comparison stops here C C H Z-configuration Copyright © 2010 Pearson Education, Inc. path goes to C, not H
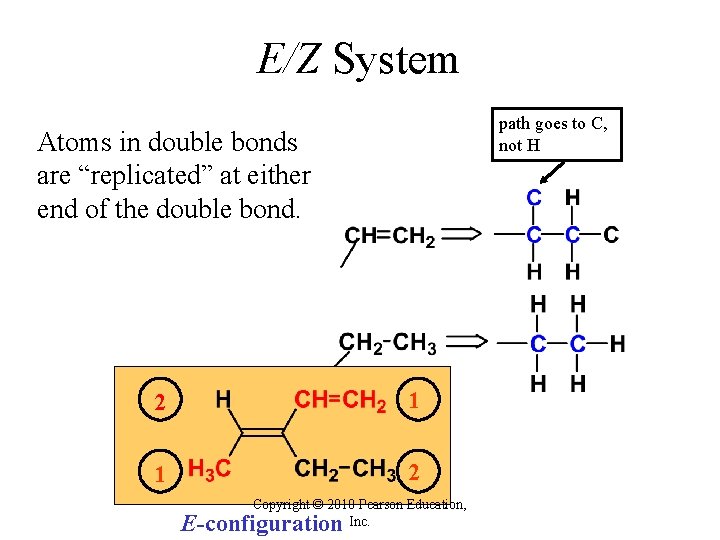
E/Z System path goes to C, not H Atoms in double bonds are “replicated” at either end of the double bond. 2 1 1 2 Copyright © 2010 Pearson Education, Inc. E-configuration
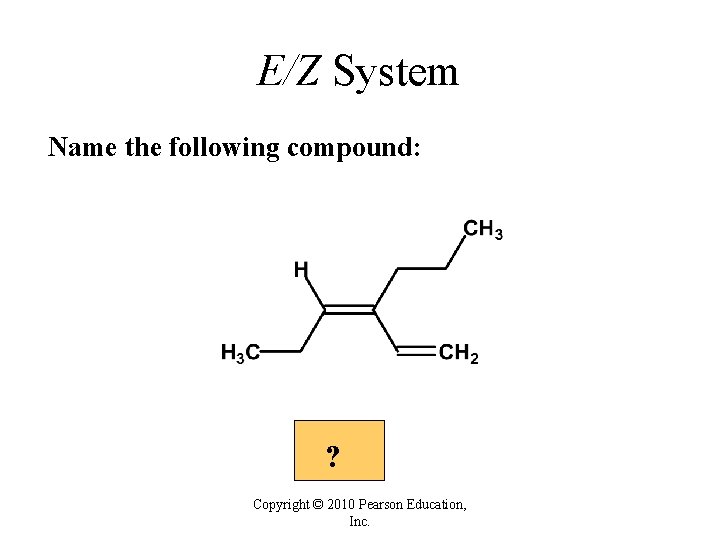
E/Z System Name the following compound: ? Copyright © 2010 Pearson Education, Inc.
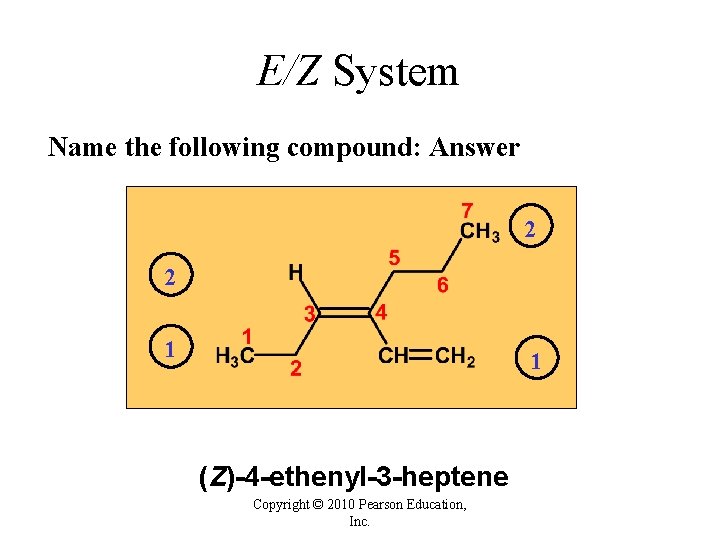
E/Z System Name the following compound: Answer 2 2 1 1 (Z)-4 -ethenyl-3 -heptene Copyright © 2010 Pearson Education, Inc.
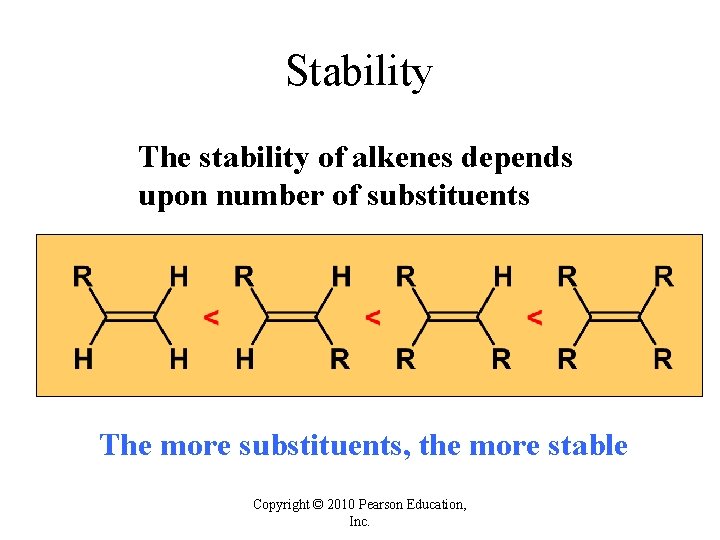
Stability The stability of alkenes depends upon number of substituents The more substituents, the more stable Copyright © 2010 Pearson Education, Inc.
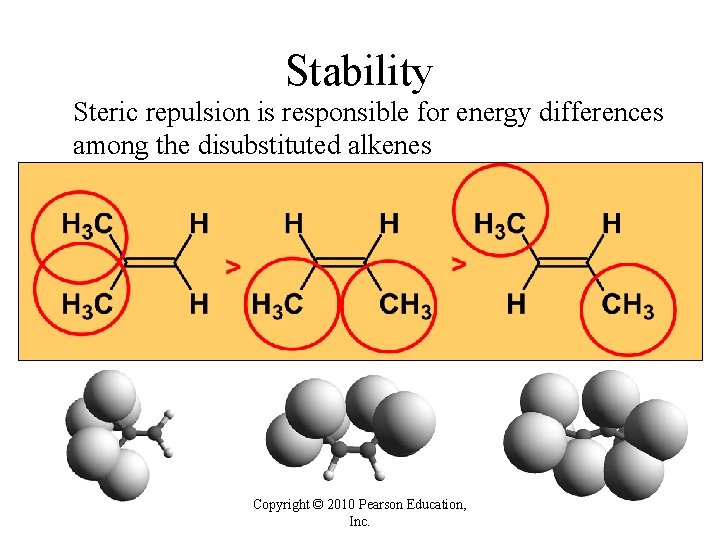
Stability Steric repulsion is responsible for energy differences among the disubstituted alkenes Copyright © 2010 Pearson Education, Inc.
Reactions Ø Alkenes are similar in structure and do similar reactions. • All contain a double bond • All contain the same functional group Ø Reactions are categorized through different types of mechanisms. Copyright © 2010 Pearson Education, Inc.
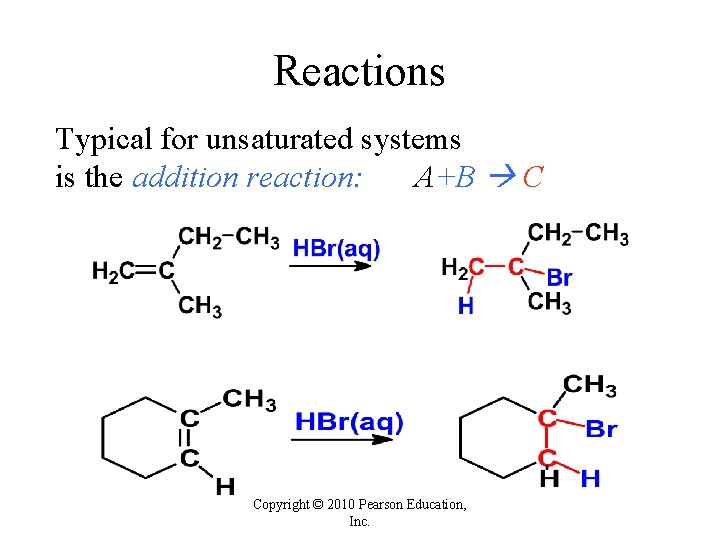
Reactions Typical for unsaturated systems is the addition reaction: A+B C Copyright © 2010 Pearson Education, Inc.

Reactions A LOOK AT THE REACTANTS Copyright © 2010 Pearson Education, Inc.
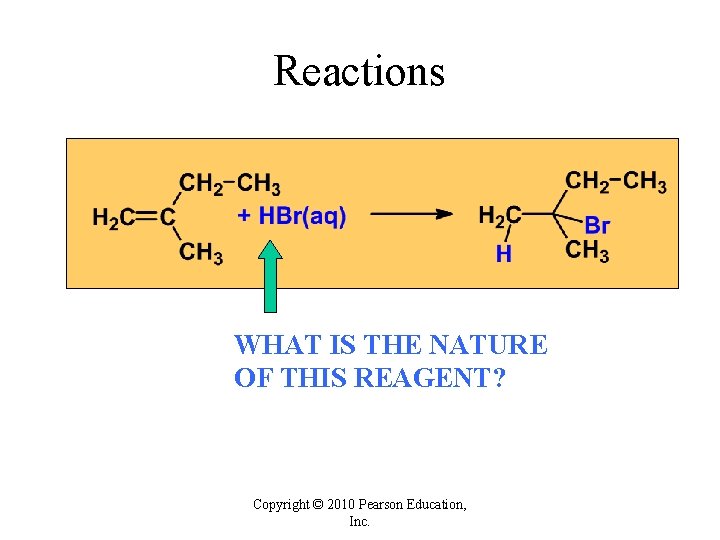
Reactions WHAT IS THE NATURE OF THIS REAGENT? Copyright © 2010 Pearson Education, Inc.
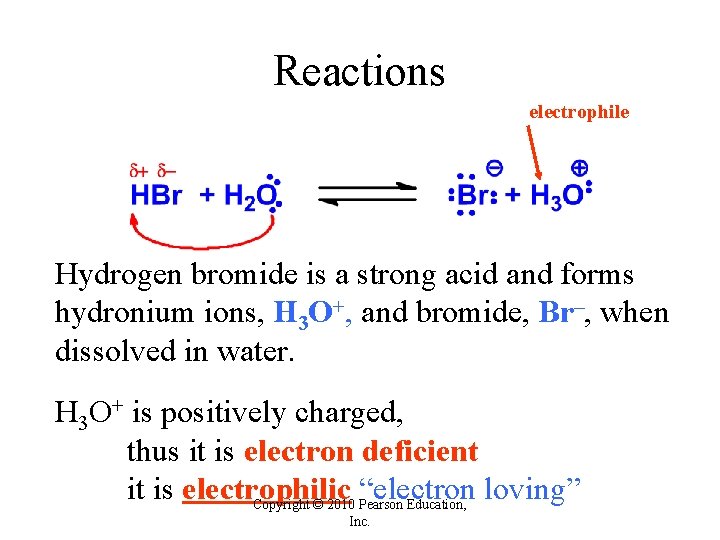
Reactions electrophile Hydrogen bromide is a strong acid and forms hydronium ions, H 3 O+, and bromide, Br–, when dissolved in water. H 3 O+ is positively charged, thus it is electron deficient it is electrophilic “electron loving” Copyright © 2010 Pearson Education, Inc.
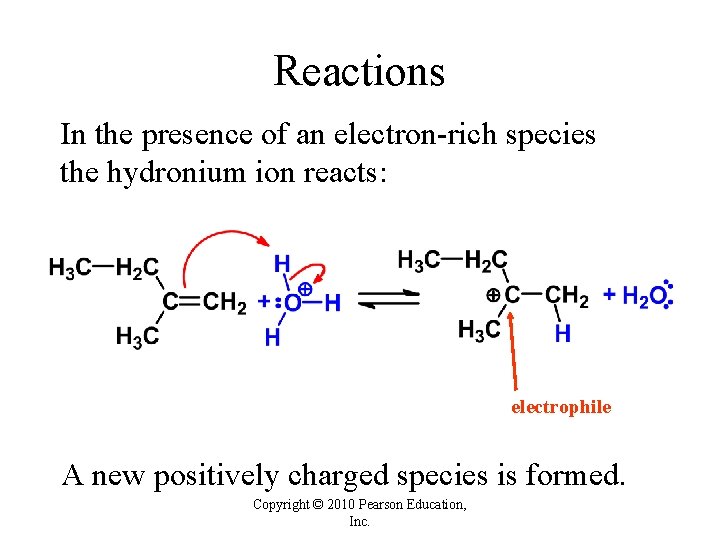
Reactions In the presence of an electron-rich species the hydronium ion reacts: electrophile A new positively charged species is formed. Copyright © 2010 Pearson Education, Inc.
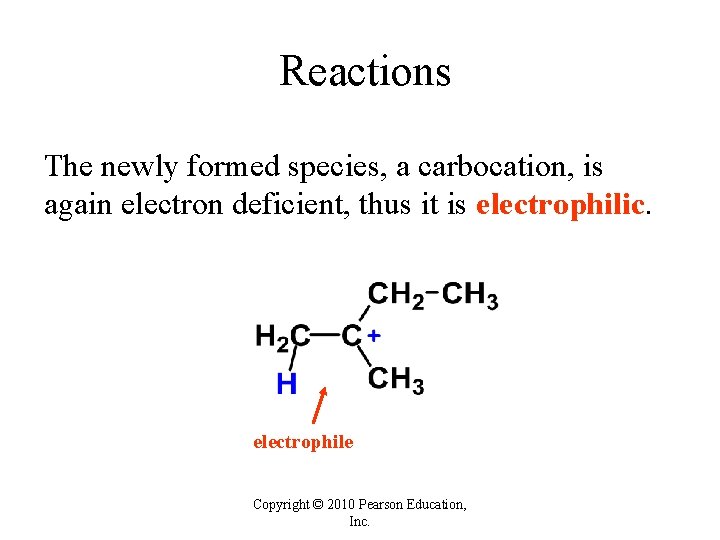
Reactions The newly formed species, a carbocation, is again electron deficient, thus it is electrophilic. electrophile Copyright © 2010 Pearson Education, Inc.
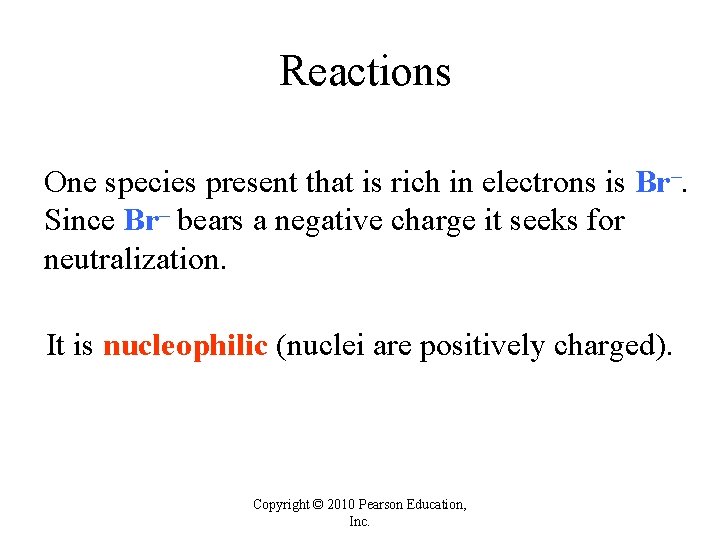
Reactions One species present that is rich in electrons is Br–. Since Br– bears a negative charge it seeks for neutralization. It is nucleophilic (nuclei are positively charged). Copyright © 2010 Pearson Education, Inc.
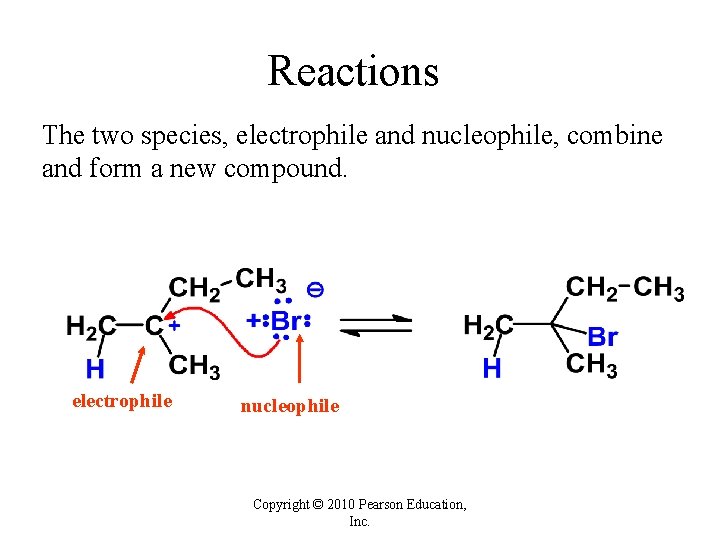
Reactions The two species, electrophile and nucleophile, combine and form a new compound. electrophile nucleophile Copyright © 2010 Pearson Education, Inc.
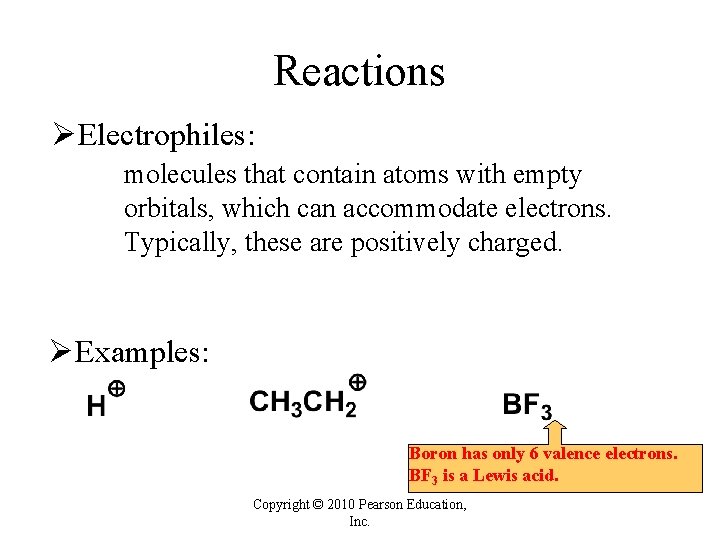
Reactions ØElectrophiles: molecules that contain atoms with empty orbitals, which can accommodate electrons. Typically, these are positively charged. ØExamples: Boron has only 6 valence electrons. BF 3 is a Lewis acid. Copyright © 2010 Pearson Education, Inc.
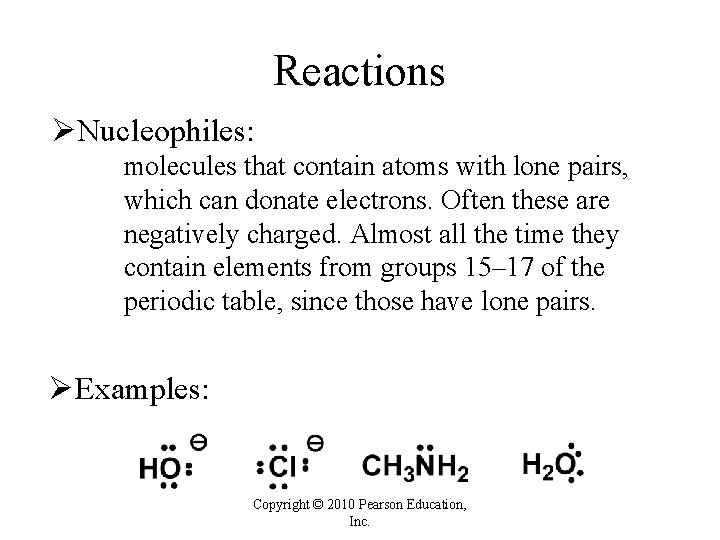
Reactions ØNucleophiles: molecules that contain atoms with lone pairs, which can donate electrons. Often these are negatively charged. Almost all the time they contain elements from groups 15– 17 of the periodic table, since those have lone pairs. ØExamples: Copyright © 2010 Pearson Education, Inc.
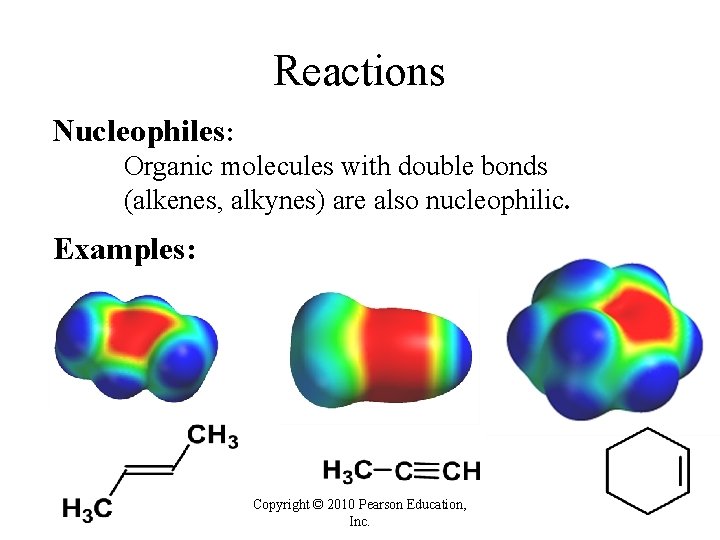
Reactions Nucleophiles: Organic molecules with double bonds (alkenes, alkynes) are also nucleophilic. Examples: Copyright © 2010 Pearson Education, Inc.
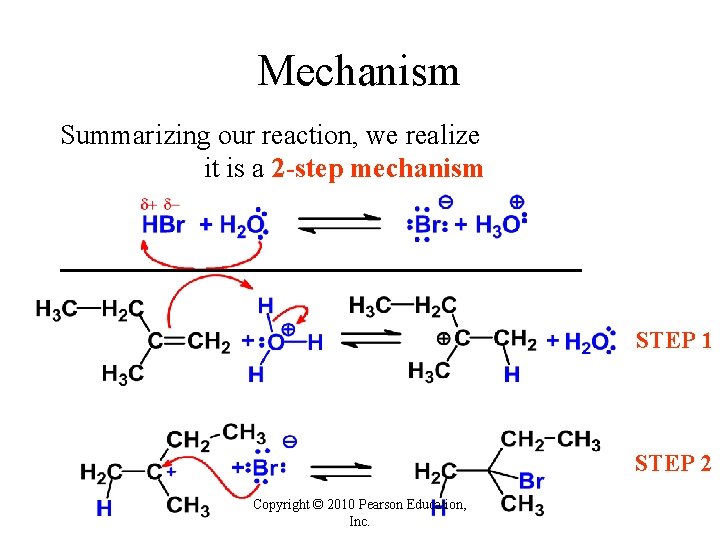
Mechanism Summarizing our reaction, we realize it is a 2 -step mechanism STEP 1 STEP 2 Copyright © 2010 Pearson Education, Inc.
Mechanism Step 1 reaches a carbocation “intermediate. ” One new bond is formed. Intermediates are species with a very short lifetime. However, their stability (energy) often determines the outcome of a reaction. Step 2 completes the reaction by forming a second bond. Again, it is the interplay between positively charged (electrophilic) and negatively charged (nucleophilic) species. Copyright © 2010 Pearson Education, Inc.
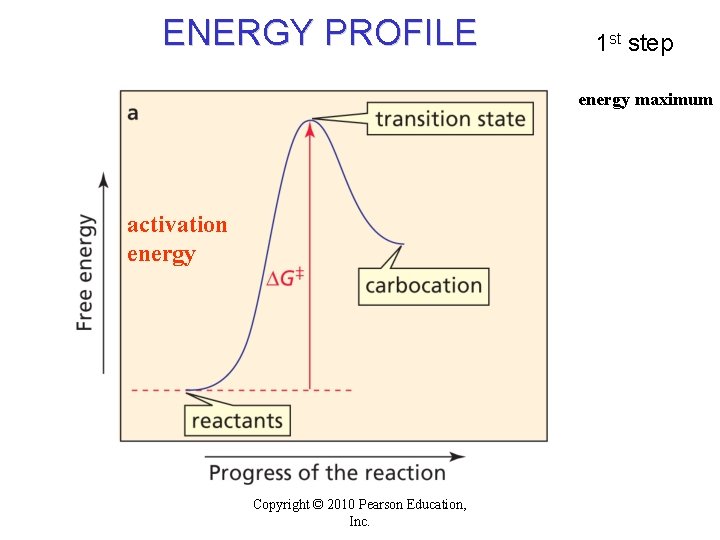
ENERGY PROFILE 1 st step energy maximum activation energy Copyright © 2010 Pearson Education, Inc.
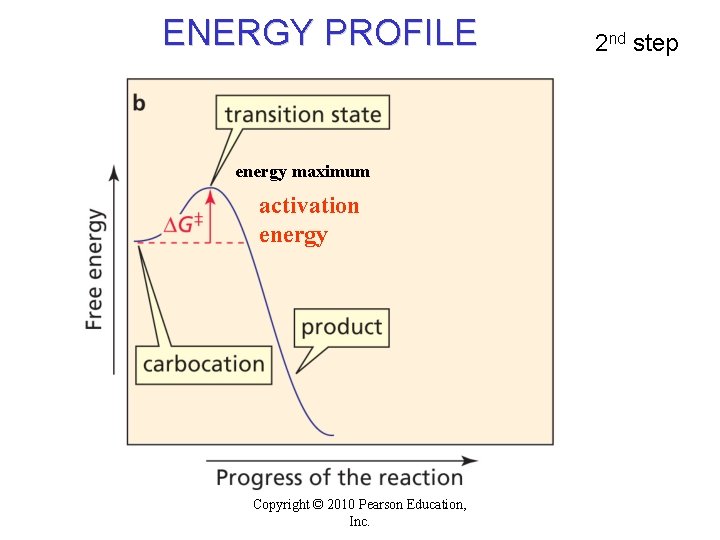
ENERGY PROFILE energy maximum activation energy Copyright © 2010 Pearson Education, Inc. 2 nd step
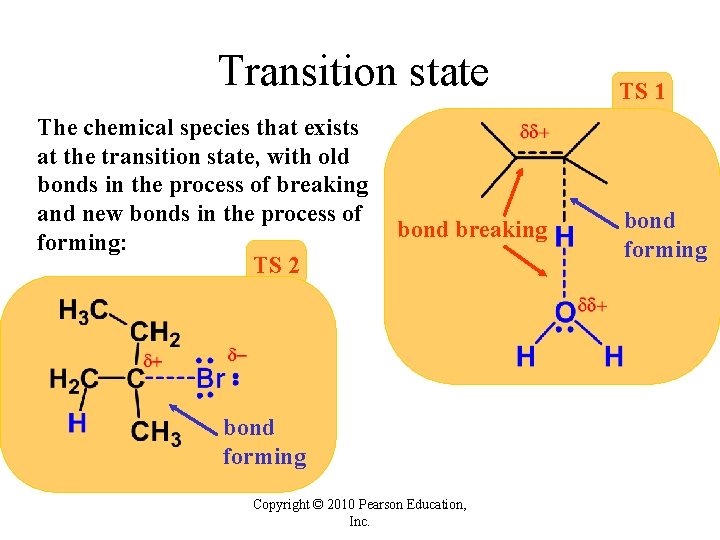
Transition state The chemical species that exists at the transition state, with old bonds in the process of breaking and new bonds in the process of forming: TS 2 bond breaking bond forming Copyright © 2010 Pearson Education, Inc. TS 1 bond forming
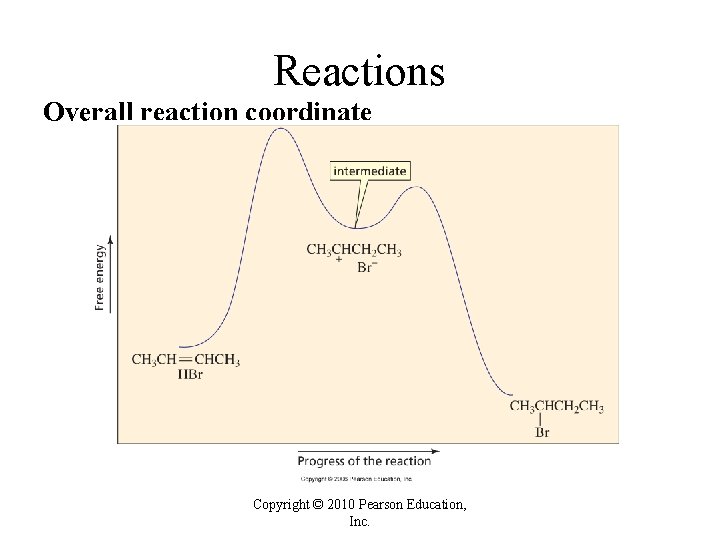
Reactions Overall reaction coordinate Copyright © 2010 Pearson Education, Inc.
- Slides: 51