ESPRIT BTK Development and Status of BRS Clinical

ESPRIT BTK Development and Status of BRS Clinical Trials for Endovascular Applications Richard J. Rapoza, Ph. D DVP of Global Clinical Affairs Abbott Vascular Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.

Richard J. Rapoza, Ph. D Full time employment: Abbott Vascular Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.

CLI challenges and treatment objectives Patients with CLI are often faced with the grim possibility of limb amputation due to non-healing wounds leading to gangrene and infection • Amputations are associated with 40% mortality rate at 1 year 1 • Only 40% regain mobility 2 Goals of revascularization: • Acute: – – • Maximize/Restore in-line flow to the distal vasculature to improve perfusion of ischemic tissue Minimize acute vessel recoil Long term: – – – Enhance wound-healing Maintain patency to prevent recurrence and need for re-intervention Enable future re-treatment if necessary 1. Duff, S. , et al. Vasc Health and Risk Mgt. 2019; 15: 187 -208. 2. Norgren, L. J Vasc Surg. 2007; 45: S 5 -S 67 Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.
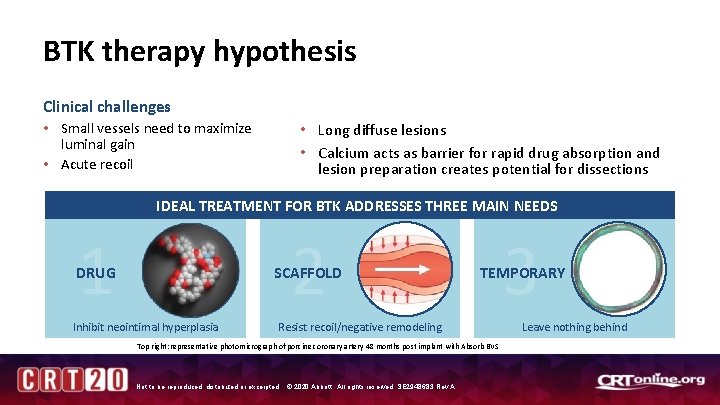
BTK therapy hypothesis Clinical challenges • Small vessels need to maximize luminal gain • Acute recoil • Long diffuse lesions • Calcium acts as barrier for rapid drug absorption and lesion preparation creates potential for dissections IDEAL TREATMENT FOR BTK ADDRESSES THREE MAIN NEEDS 1 2 DRUG SCAFFOLD Inhibit neointimal hyperplasia Resist recoil/negative remodeling Top right: representative photomicrograph of porcine coronary artery 48 months post implant with Absorb BVS Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A. 3 TEMPORARY Leave nothing behind
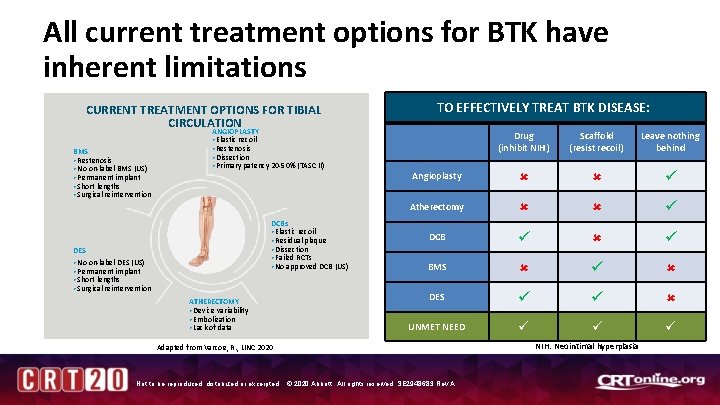
All current treatment options for BTK have inherent limitations CURRENT TREATMENT OPTIONS FOR TIBIAL CIRCULATION BMS • Restenosis • No on-label BMS (US) • Permanent implant • Short lengths • Surgical reintervention ANGIOPLASTY • Elastic recoil • Restenosis • Dissection • Primary patency 20 -50% (TASC II) DCBs • Elastic recoil • Residual plaque • Dissection • Failed RCTs • No approved DCB (US) DES • No on-label DES (US) • Permanent implant • Short lengths • Surgical reintervention ATHERECTOMY • Device variability • Embolization • Lack of data TO EFFECTIVELY TREAT BTK DISEASE: Drug (inhibit NIH) Scaffold (resist recoil) Leave nothing behind Angioplasty Atherectomy DCB BMS DES UNMET NEED Adapted from Varcoe, R. , LINC 2020 Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A. NIH: Neointimal hyperplasia
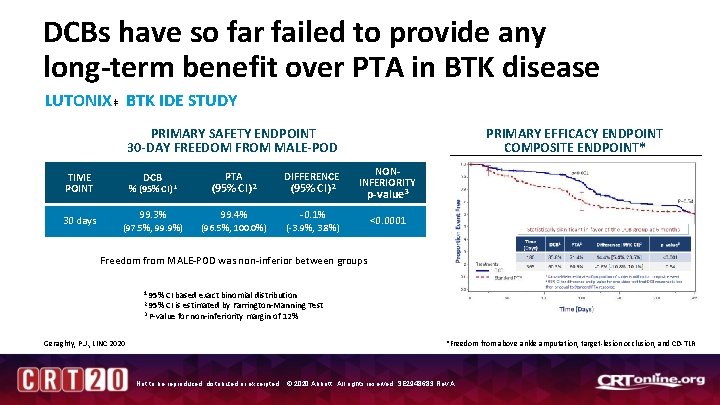
DCBs have so far failed to provide any long-term benefit over PTA in BTK disease LUTONIX ‡ BTK IDE STUDY PRIMARY SAFETY ENDPOINT 30 -DAY FREEDOM FROM MALE-POD TIME POINT 30 days DCB % (95% CI) 1 99. 3% (97. 5%, 99. 9%) PTA (95% CI) 2 99. 4% (96. 5%, 100. 0%) DIFFERENCE (95% CI) 2 PRIMARY EFFICACY ENDPOINT COMPOSITE ENDPOINT* NONINFERIORITY p-value 3 -0. 1% (-3. 9%, 3. 8%) <0. 0001 Freedom from MALE-POD was non-inferior between groups 1 95% CI based exact binomial distribution CI is estimated by Farrington-Manning Test 3 P-value for non-inferiority margin of 12% 2 95% Geraghty, P. J. , LINC 2020 *Freedom from above ankle amputation, target-lesion occlusion, and CD-TLR Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.

Meta-analysis showed benefits of DES in BTK TASC II GUIDANCE 2 “The preponderance of evidence for infrapopliteal DES, has demonstrated significant benefit over both BMS and PTA for (1) patency (2) reduced reinterventions, (3) reduced amputation, and (4) improved event-free survival. ” Vessel support (scaffolding) AND anti -proliferative drug are needed But many clinicians do not want to leave a permanent implant behind And DES are not approved for BTK in the U. S. 1. Fusaro, M. , et al. J Am Coll Cardiol Intv 2013; 6: 1284– 93. 2. TASC II Supplement, Jaff et. al. Journal of Endovascular Therapy. 2015. Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A. 1
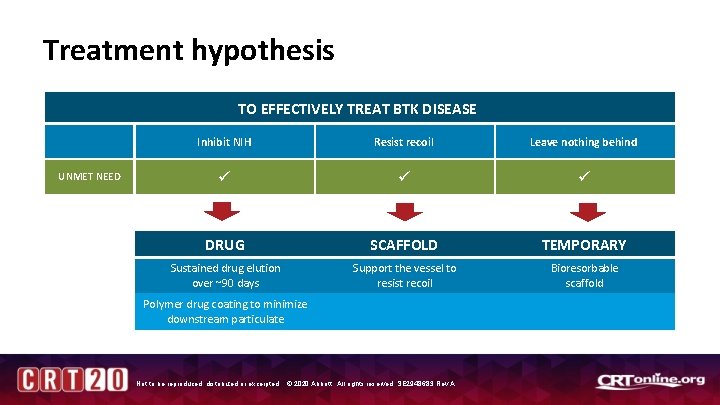
Treatment hypothesis TO EFFECTIVELY TREAT BTK DISEASE UNMET NEED Inhibit NIH Resist recoil Leave nothing behind DRUG SCAFFOLD TEMPORARY Sustained drug elution over ~90 days Support the vessel to resist recoil Bioresorbable scaffold Polymer drug coating to minimize downstream particulate Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.
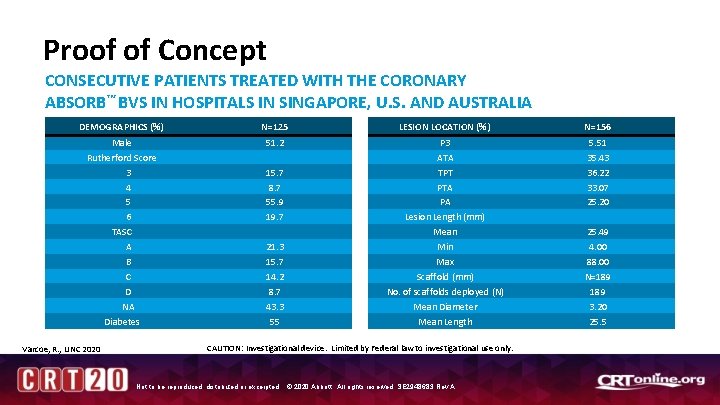
Proof of Concept CONSECUTIVE PATIENTS TREATED WITH THE CORONARY ABSORB™ BVS IN HOSPITALS IN SINGAPORE, U. S. AND AUSTRALIA DEMOGRAPHICS (%) N=125 LESION LOCATION (%) N=156 Male Rutherford Score 3 4 5 6 TASC A B C D NA Diabetes 51. 2 P 3 ATA TPT PTA PA Lesion Length (mm) Mean Min Max Scaffold (mm) No. of scaffolds deployed (N) Mean Diameter Mean Length 5. 51 35. 43 36. 22 33. 07 25. 20 Varcoe, R. , LINC 2020 15. 7 8. 7 55. 9 19. 7 21. 3 15. 7 14. 2 8. 7 43. 3 55 CAUTION: Investigational device. Limited by Federal law to investigational use only. Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A. 25. 49 4. 00 88. 00 N=189 3. 20 25. 5
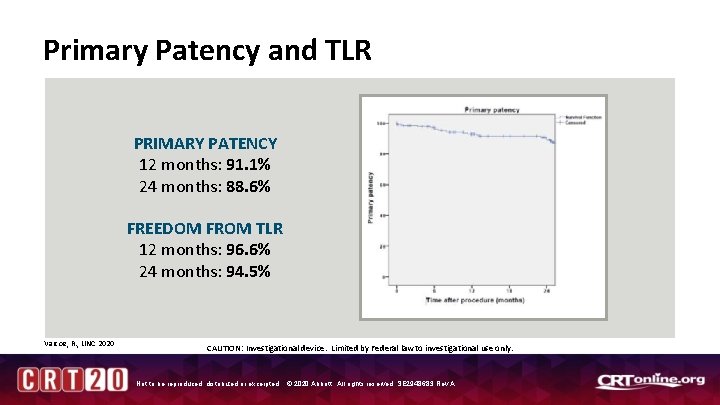
Primary Patency and TLR PRIMARY PATENCY 12 months: 91. 1% 24 months: 88. 6% FREEDOM FROM TLR 12 months: 96. 6% 24 months: 94. 5% Varcoe, R. , LINC 2020 CAUTION: Investigational device. Limited by Federal law to investigational use only. Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.
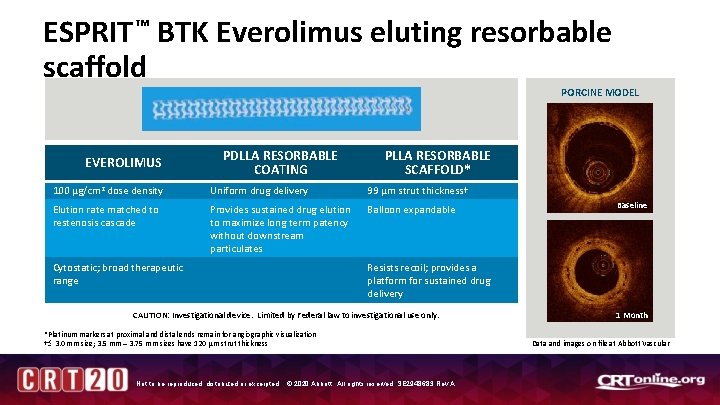
ESPRIT ™ BTK Everolimus eluting resorbable scaffold PORCINE MODEL EVEROLIMUS PDLLA RESORBABLE COATING PLLA RESORBABLE SCAFFOLD* 100 µg/cm 2 dose density Uniform drug delivery 99 µm strut thickness† Elution rate matched to restenosis cascade Provides sustained drug elution to maximize long term patency without downstream particulates Balloon expandable Cytostatic; broad therapeutic range Baseline Resists recoil; provides a platform for sustained drug delivery CAUTION: Investigational device. Limited by Federal law to investigational use only. *Platinum markers at proximal and distal ends remain for angiographic visualization † 3. 0 mm size; 3. 5 mm – 3. 75 mm sizes have 120 µm strut thickness Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A. 1 Month Data and images on file at Abbott Vascular

LIFE-BTK randomized multicenter trial PIVOTAL INVESTIGATION OF SA FETY AND EFFICACY OF ESPIRIT ™ BTK TREATMENT – BELOW THE KNEE Prospective, randomized multicenter, US and OUS single-blind, trial 225 patients randomized 2: 1 ESPRIT ™ BTK vs. PTA 6 -MONTH PRIMARY ENDPOINTS Safety Endpoint: MALE+POD Efficacy Endpoint: Primary Patency + Limb Salvage 5 -YEAR FOLLOW-UP TRIAL LEADERSHIP Ramon Varcoe MBBS, MS, FRACS, Ph. D; Sahil Parikh MD, FACC, FSCAI; Brian De. Rubertis MD, FACS CAUTION: Investigational device. Limited by Federal law to investigational use only. Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.
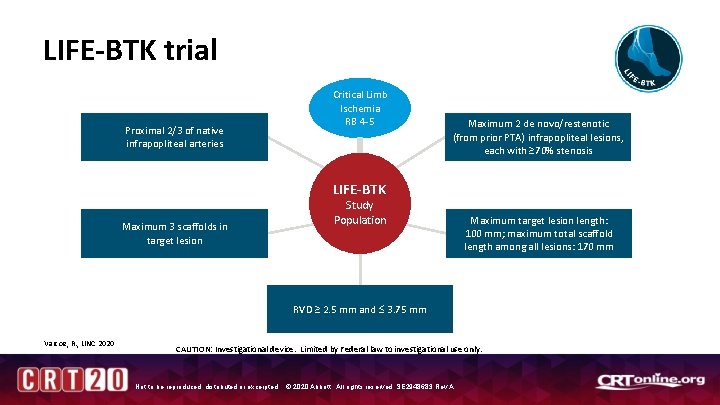
LIFE-BTK trial Proximal 2/3 of native infrapopliteal arteries Critical Limb Ischemia RB 4 -5 Maximum 2 de novo/restenotic (from prior PTA) infrapopliteal lesions, each with ≥ 70% stenosis LIFE-BTK Maximum 3 scaffolds in target lesion Study Population Maximum target lesion length: 100 mm; maximum total scaffold length among all lesions: 170 mm RVD ≥ 2. 5 mm and 3. 75 mm Varcoe, R. , LINC 2020 CAUTION: Investigational device. Limited by Federal law to investigational use only. Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.
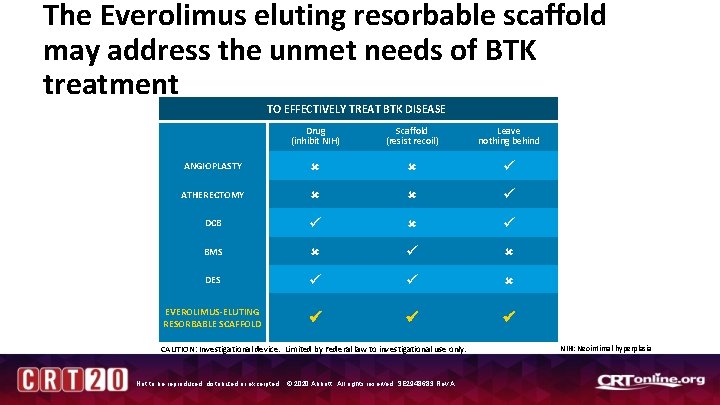
The Everolimus eluting resorbable scaffold may address the unmet needs of BTK treatment TO EFFECTIVELY TREAT BTK DISEASE Drug (inhibit NIH) Scaffold (resist recoil) Leave nothing behind ANGIOPLASTY ATHERECTOMY DCB BMS DES EVEROLIMUS-ELUTING RESORBABLE SCAFFOLD CAUTION: Investigational device. Limited by Federal law to investigational use only. Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A. NIH: Neointimal hyperplasia

CAUTION: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use inside the product carton (when available) or at eifu. abbottvascular. com or at medical. abbott/manuals for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events. Illustrations are artist’s representations only and should not be considered as engineering drawings or photographs. Photo(s) on file at Abbott 3200 Lakeside Dr. , Santa Clara, CA. 95054 USA, Tel: 1. 800. 227. 9902 ™ Indicates a trademark of the Abbott Group of Companies. ‡ Indicates a third party trademark, which is property of its respective owner. www. cardiovascular. abbott © 2020 Abbott. All rights reserved. SE 2948683 Rev. A Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.

Thank you Not to be reproduced, distributed or excerpted. © 2020 Abbott. All rights reserved. SE 2948683 Rev A.
- Slides: 16