ERT 2064 Thermodynamics CHAPTER 3 Volumetric Properties of









- Slides: 39

ERT 206/4 Thermodynamics CHAPTER 3 Volumetric Properties of Pure Fluids Miss. Rahimah Bt. Othman Email: rahimah@unimap. edu. my

COURSE OUTCOME 1 CO 1) 1. Chapter 1: Introduction to Thermodynamics 2. Chapter 2: The First Law and Other Basic Concepts 2. Chapter 3: Volumetric properties of pure fluids DESCRIBE and EXPLAIN PVT behavior of pure substances, Virial Equation of State, Ideal Gas, Virial Equation- APPLICATION, Cubic Equation of State, Generalized Correlations for gases and liquids. 4. Chapter 4: Heat effects 5. Chapter 5: Second law of thermodynamics 6. Chapter 6: Thermodynamics properties of fluids

4. Property Diagrams for Phase-Change Processes 5. Property Tables 6. The Ideal-Gas Equation of State 3. Phase. Change of Pure Substances 2. Phases of a Pure Substance PURE SUBSTANCE 1. Pure Substance 7. Compressibility Factor- A Measure of Deviation from Ideal-Gas Behavior 8. Other Equations of State

§ A substance that has a fixed chemical composition throughout is called a Pure Substance. § Pure Substance: - N 2, O 2, gaseous Air -A mixture of liquid and gaseous water is a pure substance, but a mixture of liquid and gaseous air is not.

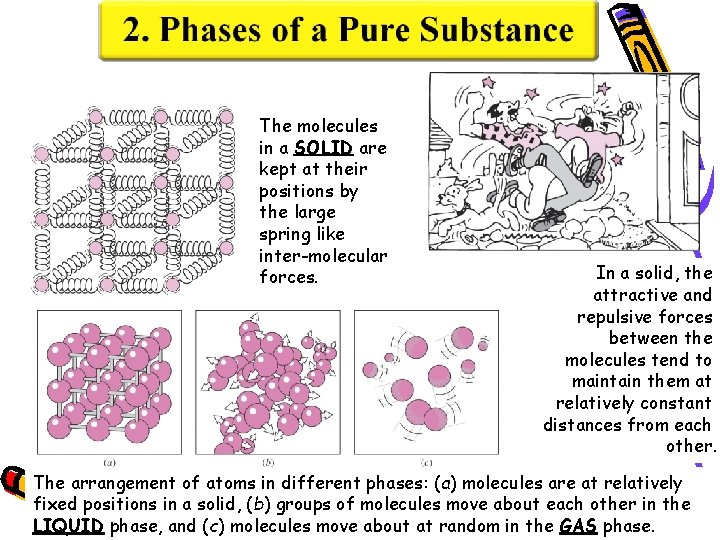
The molecules in a SOLID are kept at their positions by the large spring like inter-molecular forces. In a solid, the attractive and repulsive forces between the molecules tend to maintain them at relatively constant distances from each other. The arrangement of atoms in different phases: (a) molecules are at relatively fixed positions in a solid, (b) groups of molecules move about each other in the LIQUID phase, and (c) molecules move about at random in the GAS phase.

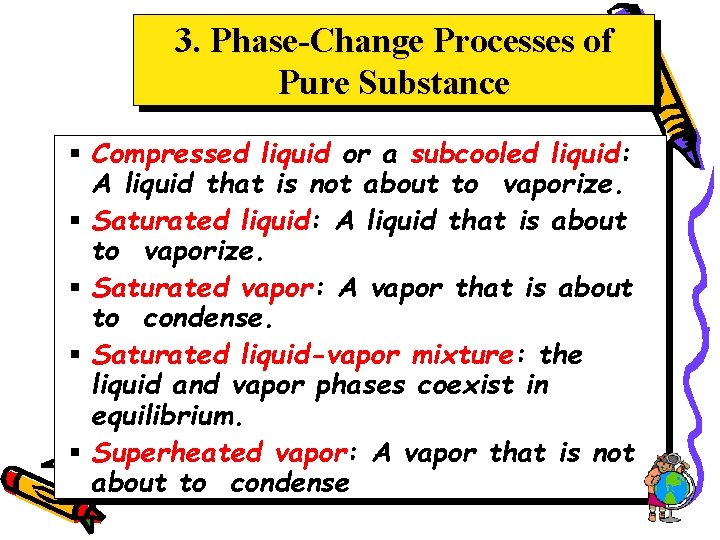
3. Phase-Change Processes of Pure Substance § Compressed liquid or a subcooled liquid: A liquid that is not about to vaporize. § Saturated liquid: A liquid that is about to vaporize. § Saturated vapor: A vapor that is about to condense. § Saturated liquid-vapor mixture: the liquid and vapor phases coexist in equilibrium. § Superheated vapor: A vapor that is not about to condense

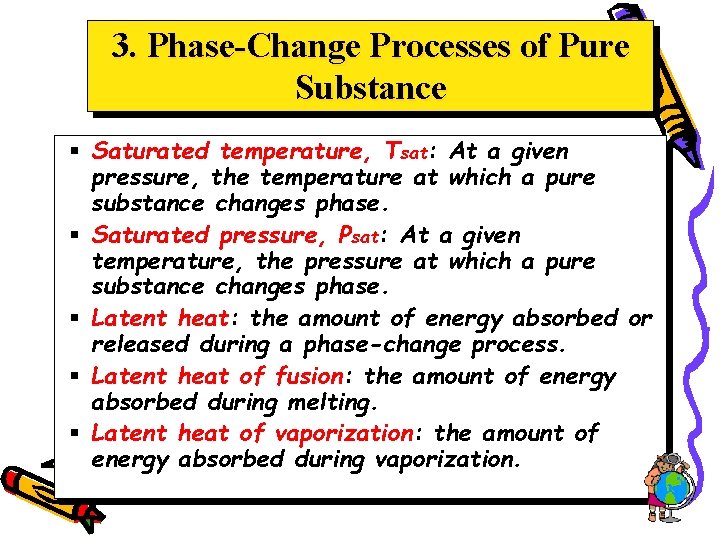
3. Phase-Change Processes of Pure Substance § Saturated temperature, Tsat: At a given pressure, the temperature at which a pure substance changes phase. § Saturated pressure, Psat: At a given temperature, the pressure at which a pure substance changes phase. § Latent heat: the amount of energy absorbed or released during a phase-change process. § Latent heat of fusion: the amount of energy absorbed during melting. § Latent heat of vaporization: the amount of energy absorbed during vaporization.

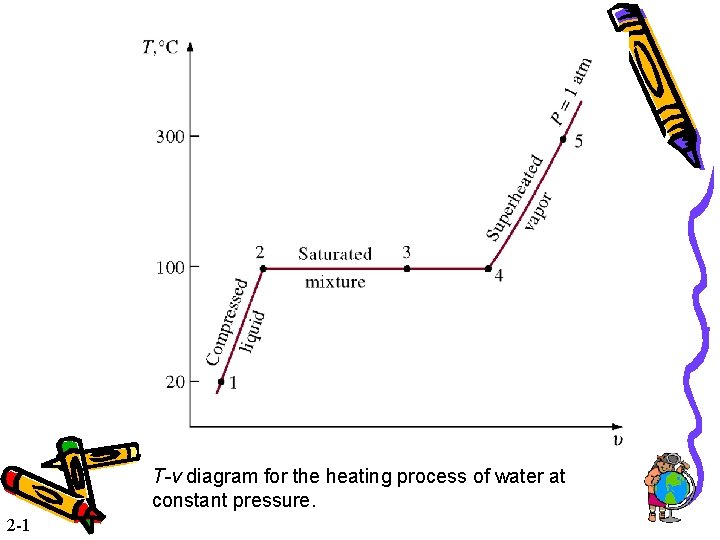
T-v diagram for the heating process of water at constant pressure. 2 -1

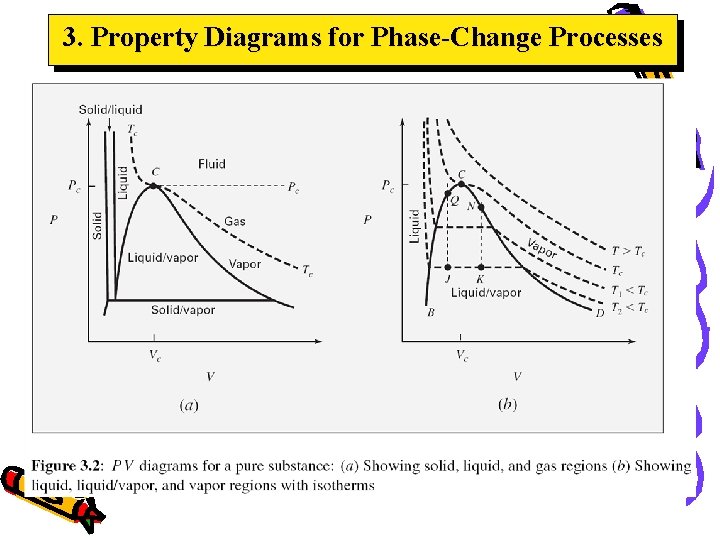
3. Property Diagrams for Phase-Change Processes Critical point – highest combination of pressure PVT BEHAVIOR OF PURE SUBSTANCES/ FLUIDSwhere the and temperature fluid exist in liq-vap equilibrium The 2 -C line, also known as vaporization curve is where liquid-vapor is in equilibrium The 1 -2 line, also known as sublimation curve is where solidvapor is in equilibrium Triple point, three phases exist in equilibrium (F=0) The 2 -3 line, also known as fusion curve is where solidliquid is in equilibrium

3. Property Diagrams for Phase-Change Processes

4. Property Tables § For most substances, the relationships among thermodynamics properties are too complex to expressed by simple equations. Therefore properties are presented in the form of tables (Property Tables). [ Smith et al. (2005); Cengel & Boles (2002)] § Property tables are given in the Appendix in both SI and English units. § The tables in English units carry the same number as the corresponding tables in SI, followed by an identifier E. § For example: Tables A-6 and A 6 -E list properties of superheated water vapor. [Tables F-2 in Smith et al. ] § Table A-4 until A-8: show internal energy (u), enthalpy (h) and entropy (s).

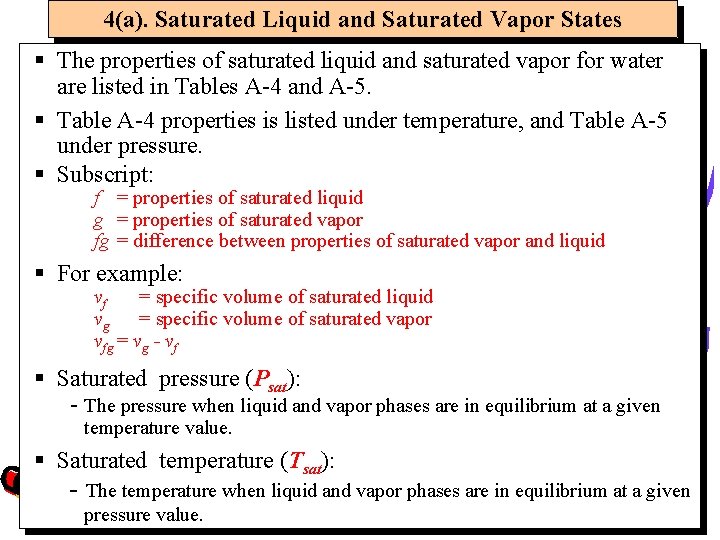
4(a). Saturated Liquid and Saturated Vapor States § The properties of saturated liquid and saturated vapor for water are listed in Tables A-4 and A-5. § Table A-4 properties is listed under temperature, and Table A-5 under pressure. § Subscript: f = properties of saturated liquid g = properties of saturated vapor fg = difference between properties of saturated vapor and liquid § For example: vf = specific volume of saturated liquid vg = specific volume of saturated vapor vfg = vg - vf § Saturated pressure (Psat): - The pressure when liquid and vapor phases are in equilibrium at a given temperature value. § Saturated temperature (Tsat): - The temperature when liquid and vapor phases are in equilibrium at a given pressure value.

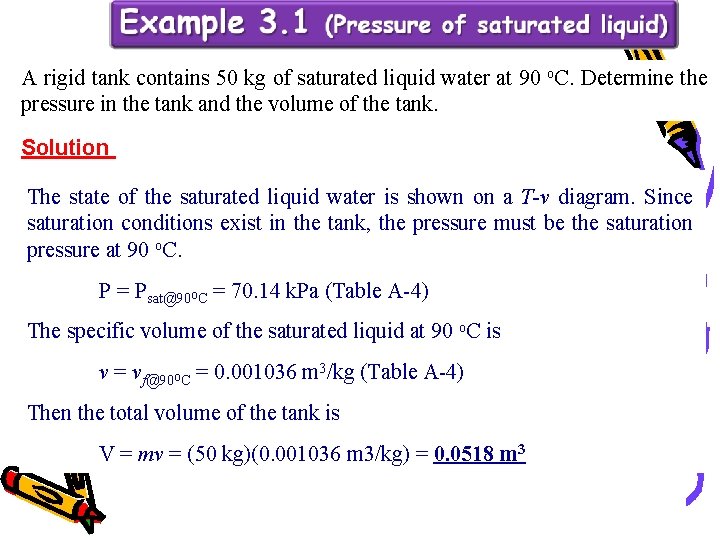
A rigid tank contains 50 kg of saturated liquid water at 90 o. C. Determine the pressure in the tank and the volume of the tank. Solution The state of the saturated liquid water is shown on a T-v diagram. Since saturation conditions exist in the tank, the pressure must be the saturation pressure at 90 o. C. P = Psat@90 o. C = 70. 14 k. Pa (Table A-4) The specific volume of the saturated liquid at 90 o. C is v = vf@90 o. C = 0. 001036 m 3/kg (Table A-4) Then the total volume of the tank is V = mv = (50 kg)(0. 001036 m 3/kg) = 0. 0518 m 3

A piston-cylinder device contains 2 ft 3 of saturated water vapor at 50 -psia pressure. Determine the temperature and the mass of the vapor inside the cylinder. Solution The state of the saturated water vapor is shown on a P-v diagram. Since the cylinder contains saturated vapor at 50 psia, the temperature inside must be the saturation temperature at this pressure. T = Tsat@90 o. C = 281. 03 o. F (Table A-5 E) The specific volume of the saturated vapor at 50 psia is; v = vf@50 psia = 8. 518 ft 3/lbm (Table A-5 E) Then the mass of water vapor inside the cylinder becomes;

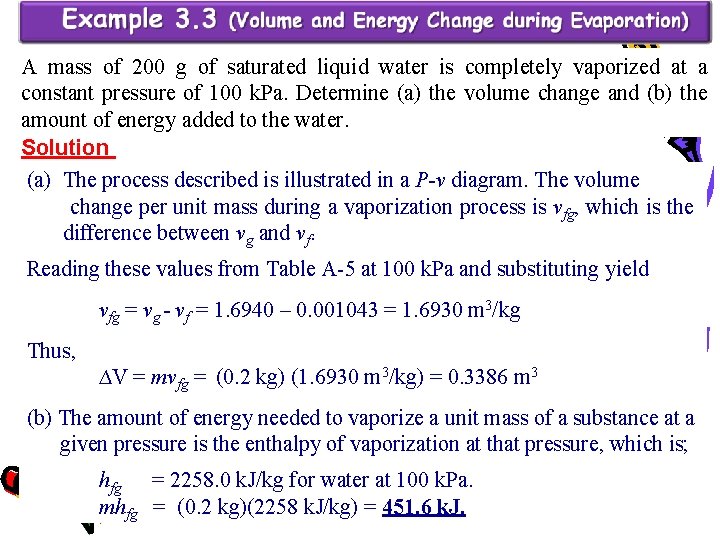
A mass of 200 g of saturated liquid water is completely vaporized at a constant pressure of 100 k. Pa. Determine (a) the volume change and (b) the amount of energy added to the water. Solution (a) The process described is illustrated in a P-v diagram. The volume change per unit mass during a vaporization process is vfg, which is the difference between vg and vf. Reading these values from Table A-5 at 100 k. Pa and substituting yield vfg = vg - vf = 1. 6940 – 0. 001043 = 1. 6930 m 3/kg Thus, ∆V = mvfg = (0. 2 kg) (1. 6930 m 3/kg) = 0. 3386 m 3 (b) The amount of energy needed to vaporize a unit mass of a substance at a given pressure is the enthalpy of vaporization at that pressure, which is; hfg = 2258. 0 k. J/kg for water at 100 k. Pa. mhfg = (0. 2 kg)(2258 k. J/kg) = 451. 6 k. J.

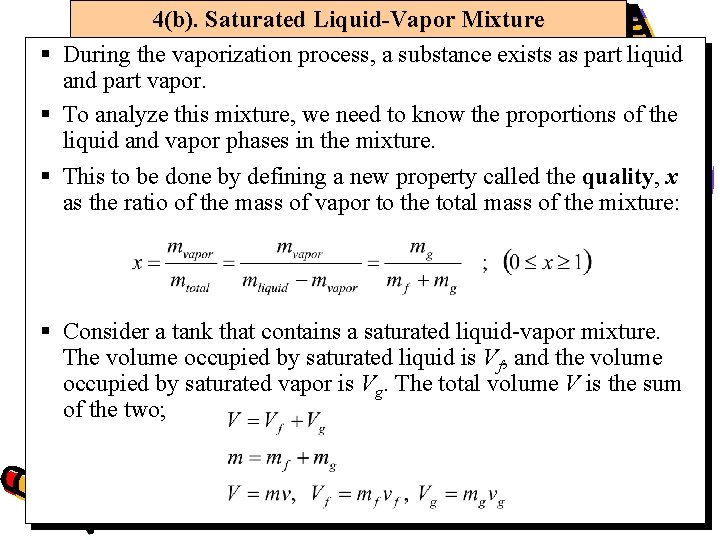
4(b). Saturated Liquid-Vapor Mixture § During the vaporization process, a substance exists as part liquid and part vapor. § To analyze this mixture, we need to know the proportions of the liquid and vapor phases in the mixture. § This to be done by defining a new property called the quality, x as the ratio of the mass of vapor to the total mass of the mixture: § Consider a tank that contains a saturated liquid-vapor mixture. The volume occupied by saturated liquid is Vf, and the volume occupied by saturated vapor is Vg. The total volume V is the sum of the two;

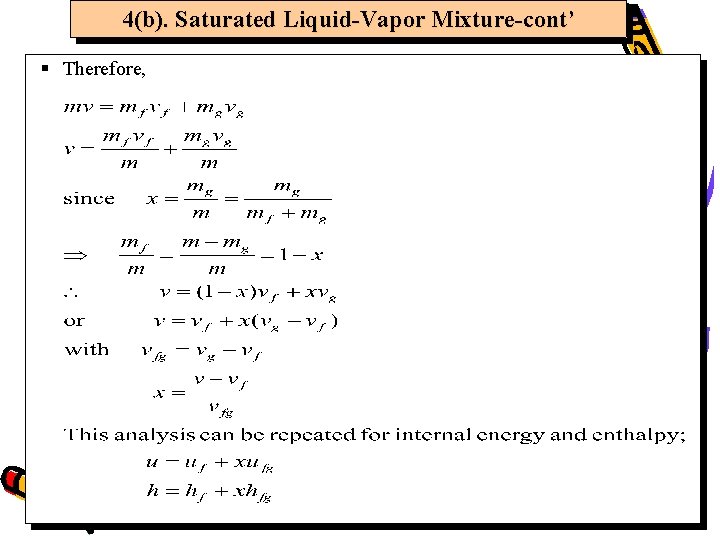
4(b). Saturated Liquid-Vapor Mixture-cont’ § Therefore,

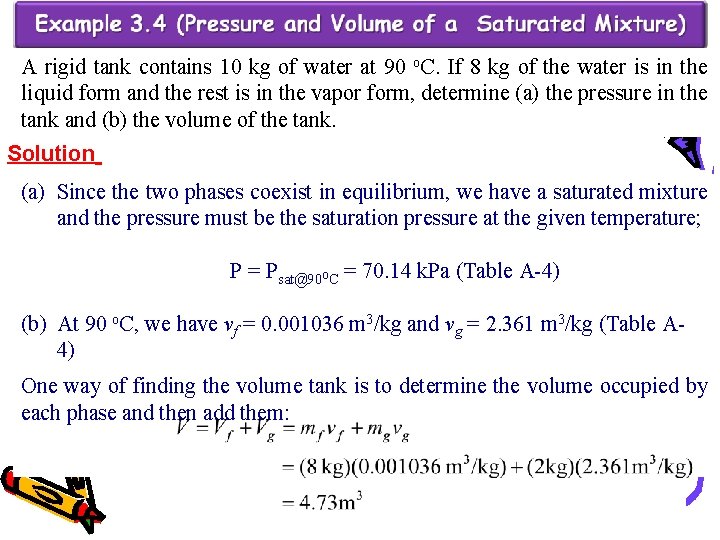
A rigid tank contains 10 kg of water at 90 o. C. If 8 kg of the water is in the liquid form and the rest is in the vapor form, determine (a) the pressure in the tank and (b) the volume of the tank. Solution (a) Since the two phases coexist in equilibrium, we have a saturated mixture and the pressure must be the saturation pressure at the given temperature; P = Psat@90 o. C = 70. 14 k. Pa (Table A-4) (b) At 90 o. C, we have vf = 0. 001036 m 3/kg and vg = 2. 361 m 3/kg (Table A 4) One way of finding the volume tank is to determine the volume occupied by each phase and then add them:

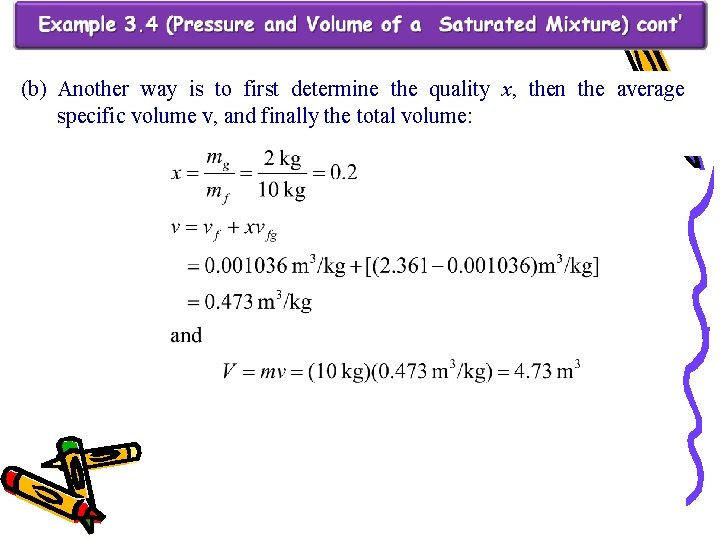
(b) Another way is to first determine the quality x, then the average specific volume v, and finally the total volume:

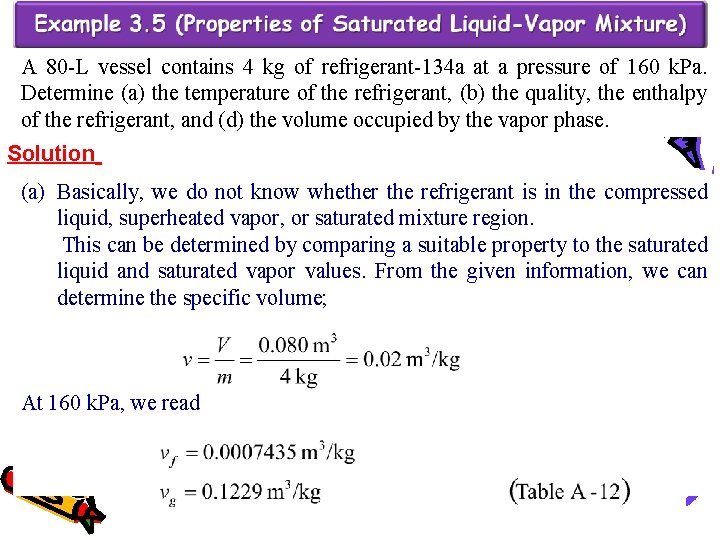
A 80 -L vessel contains 4 kg of refrigerant-134 a at a pressure of 160 k. Pa. Determine (a) the temperature of the refrigerant, (b) the quality, the enthalpy of the refrigerant, and (d) the volume occupied by the vapor phase. Solution (a) Basically, we do not know whether the refrigerant is in the compressed liquid, superheated vapor, or saturated mixture region. This can be determined by comparing a suitable property to the saturated liquid and saturated vapor values. From the given information, we can determine the specific volume; At 160 k. Pa, we read

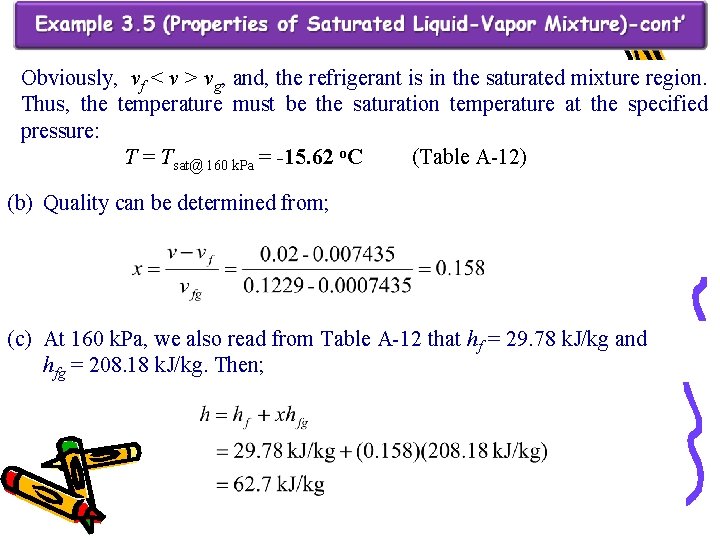
Obviously, vf ˂ v ˃ vg, and, the refrigerant is in the saturated mixture region. Thus, the temperature must be the saturation temperature at the specified pressure: T = Tsat@ 160 k. Pa = -15. 62 o. C (Table A-12) (b) Quality can be determined from; (c) At 160 k. Pa, we also read from Table A-12 that hf = 29. 78 k. J/kg and hfg = 208. 18 k. J/kg. Then;

(d) The mass of the vapor is; and the volume occupied by the vapor phase is; The rest of the volume (2. 3 L) is occupied by the liquid.

4(c). Superheated Vapor § In the region to the right of the saturated vapor line and at temperatures above the critical point temperature, a substance exists as superheated vapor. § Superheated vapor is characterized by; q. Lower pressures (P < Psat at a given T) q. Higher temperatures (T > Tsat at a given P) q. Higher specific volumes (v > vg at a given P or T) q. Higher internal energies (u > ug at a given P or T) q. Higher enthalpies (h > hg at a given P or T)

Determine the temperature of water at a state of P = 0. 5 MPa and h = 2890 k. J/kg. Solution At 0. 5 Mpa, the enthalpy of saturated water vapor is hg = 2748. 7 k. J/kg. Since h > hg, now we have superheated vapor. Under 0. 5 MPa. In Table A-6 we read; T, o. C h, k. J/kg 200 2855. 4 250 2960. 7 Obviously, the temperature is between 200 and 250 o. C. By linear interpolation it is determined to be: T = 216. 4 o. C.

4(d). Compressed Liquid § In general, a compressed liquid is characterized by; q. Higher pressures (P > Psat at a given T) q. Lower temperatures (T < Tsat at a given P) q. Lower specific volumes (v < vg at a given P or T) q. Lower internal energies (u < ug at a given P or T) q. Lower enthalpies (h < hg at a given P or T) § In the absence of compressed liquid data, a general approximation is to treat compressed liquid as saturated liquid at the given temperature. § This is because the compressed liquid properties depend on temperature much more strongly than they do on pressure.

Determine the internal energy of compressed liquid water at 80 o. C and 5 MPa, using (a) data from the compressed liquid table and (b) saturated liquid data. What is the error involved in the second case? Solution At 80 o. C, the saturation pressure of water is 47. 39 k. Pa, and since 5 MPa > Psat, we obviously have compressed liquid. (a) From the compressed liquid table (Table A-7) (b) From the saturation table (Table A-4), we read The error involved is which is less than 1 percent.

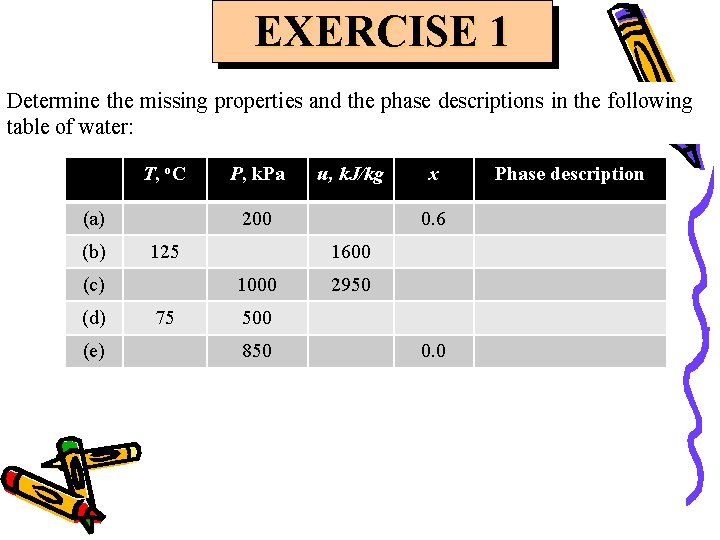
EXERCISE 1 Determine the missing properties and the phase descriptions in the following table of water: T, o. C (a) (b) (e) u, k. J/kg 200 125 (c) (d) P, k. Pa 0. 6 1600 1000 75 x 2950 500 850 0. 0 Phase description

EXERCISE 1 Determine the missing properties and the phase descriptions in the following table of water: Answer: T, o. C (a) 120. 23 P, k. Pa u, k. J/kg x Phase description 200 1719. 49 0. 6 Saturated liquid-vapor mixture (b) 125 232. 1 1600 0. 535 Saturated liquid-vapor mixture (c) 395. 6 1000 2950 No meaning Superheated vapor (d) 75 500 313. 90 No meaning Compressed liquid 850 731. 27 0. 0 Saturated liquid (e) 172. 96

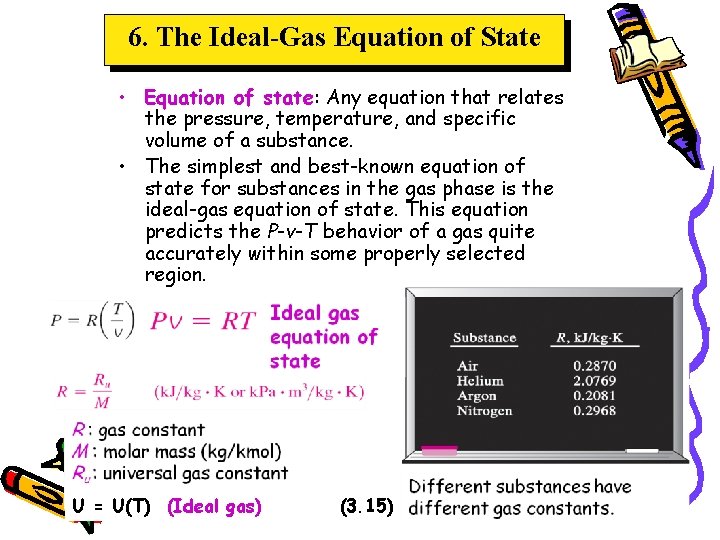
6. The Ideal-Gas Equation of State • Equation of state: Any equation that relates the pressure, temperature, and specific volume of a substance. • The simplest and best-known equation of state for substances in the gas phase is the ideal-gas equation of state. This equation predicts the P-v-T behavior of a gas quite accurately within some properly selected region. U = U(T) (Ideal gas) (3. 15)

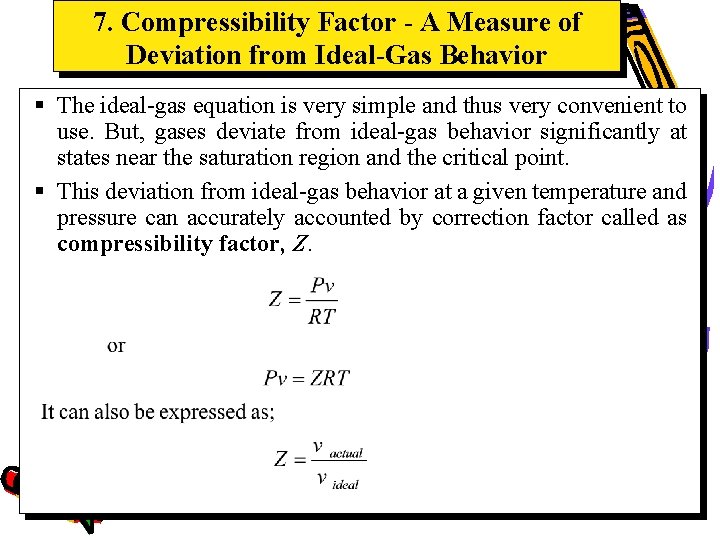
7. Compressibility Factor - A Measure of Deviation from Ideal-Gas Behavior § The ideal-gas equation is very simple and thus very convenient to use. But, gases deviate from ideal-gas behavior significantly at states near the saturation region and the critical point. § This deviation from ideal-gas behavior at a given temperature and pressure can accurately accounted by correction factor called as compressibility factor, Z.

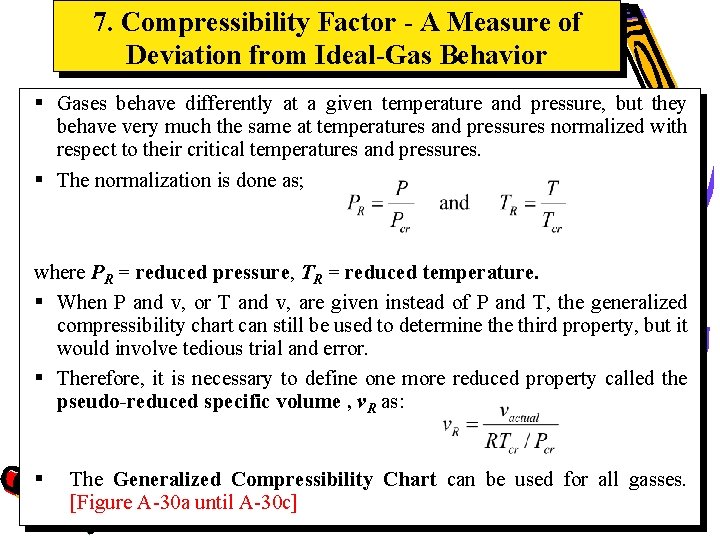
7. Compressibility Factor - A Measure of Deviation from Ideal-Gas Behavior § Gases behave differently at a given temperature and pressure, but they behave very much the same at temperatures and pressures normalized with respect to their critical temperatures and pressures. § The normalization is done as; where PR = reduced pressure, TR = reduced temperature. § When P and v, or T and v, are given instead of P and T, the generalized compressibility chart can still be used to determine third property, but it would involve tedious trial and error. § Therefore, it is necessary to define one more reduced property called the pseudo-reduced specific volume , v. R as: § The Generalized Compressibility Chart can be used for all gasses. [Figure A-30 a until A-30 c]

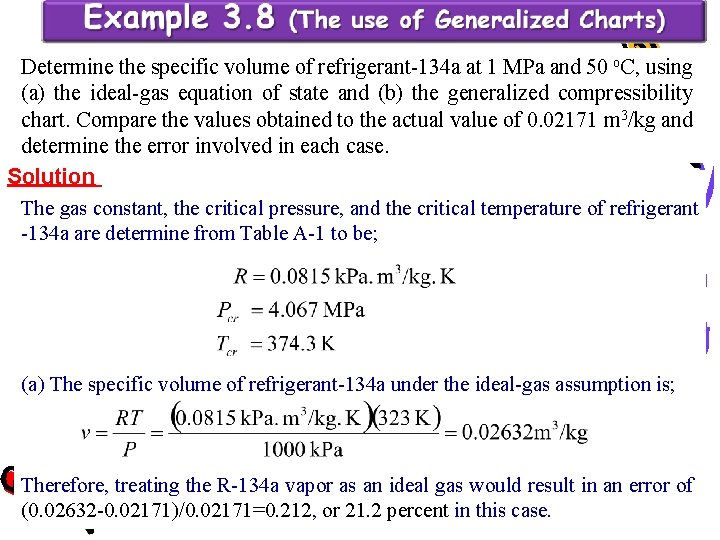
Determine the specific volume of refrigerant-134 a at 1 MPa and 50 o. C, using (a) the ideal-gas equation of state and (b) the generalized compressibility chart. Compare the values obtained to the actual value of 0. 02171 m 3/kg and determine the error involved in each case. Solution The gas constant, the critical pressure, and the critical temperature of refrigerant -134 a are determine from Table A-1 to be; (a) The specific volume of refrigerant-134 a under the ideal-gas assumption is; Therefore, treating the R-134 a vapor as an ideal gas would result in an error of (0. 02632 -0. 02171)/0. 02171=0. 212, or 21. 2 percent in this case.

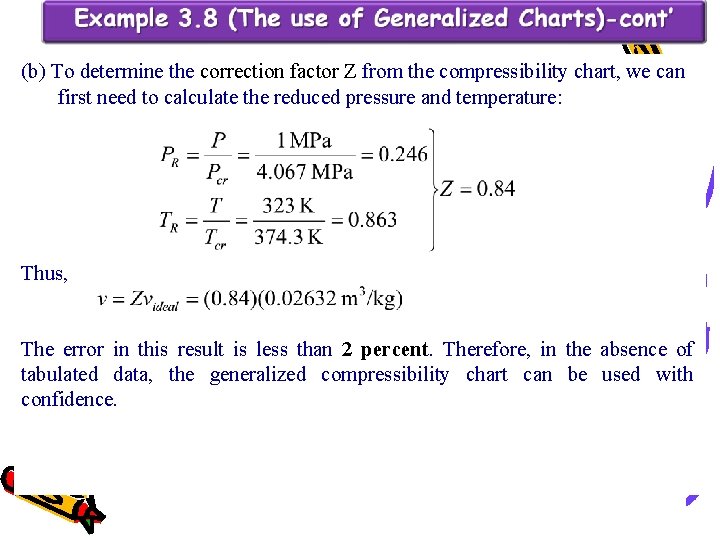
(b) To determine the correction factor Z from the compressibility chart, we can first need to calculate the reduced pressure and temperature: Thus, The error in this result is less than 2 percent. Therefore, in the absence of tabulated data, the generalized compressibility chart can be used with confidence.

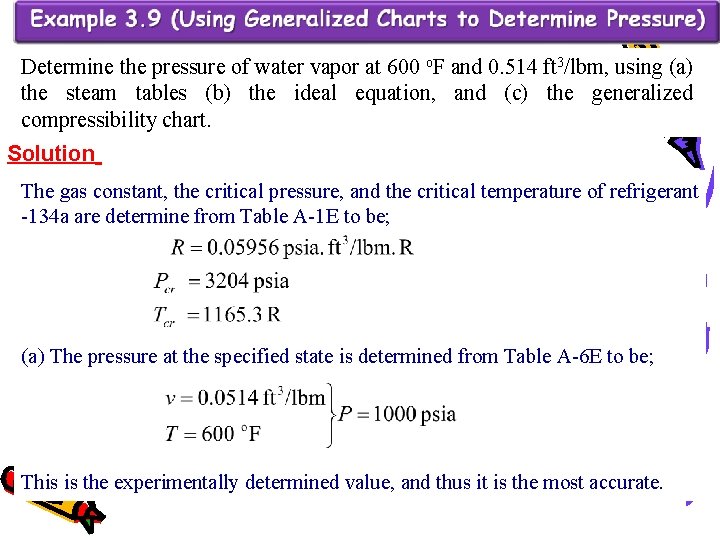
Determine the pressure of water vapor at 600 o. F and 0. 514 ft 3/lbm, using (a) the steam tables (b) the ideal equation, and (c) the generalized compressibility chart. Solution The gas constant, the critical pressure, and the critical temperature of refrigerant -134 a are determine from Table A-1 E to be; (a) The pressure at the specified state is determined from Table A-6 E to be; This is the experimentally determined value, and thus it is the most accurate.

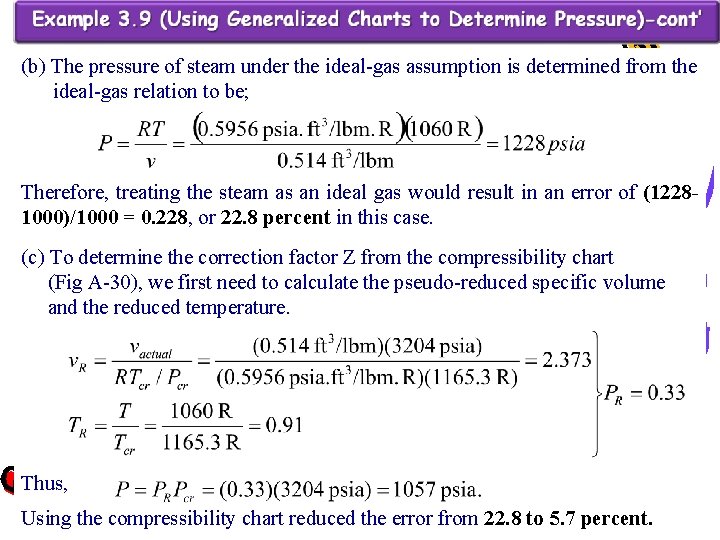
(b) The pressure of steam under the ideal-gas assumption is determined from the ideal-gas relation to be; Therefore, treating the steam as an ideal gas would result in an error of (12281000)/1000 = 0. 228, or 22. 8 percent in this case. (c) To determine the correction factor Z from the compressibility chart (Fig A-30), we first need to calculate the pseudo-reduced specific volume and the reduced temperature. Thus, Using the compressibility chart reduced the error from 22. 8 to 5. 7 percent.

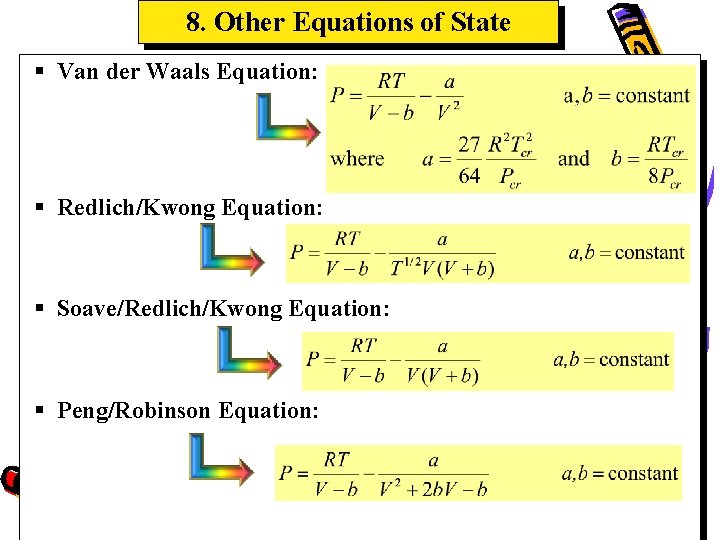
8. Other Equations of State § Van der Waals Equation: § Redlich/Kwong Equation: § Soave/Redlich/Kwong Equation: § Peng/Robinson Equation:

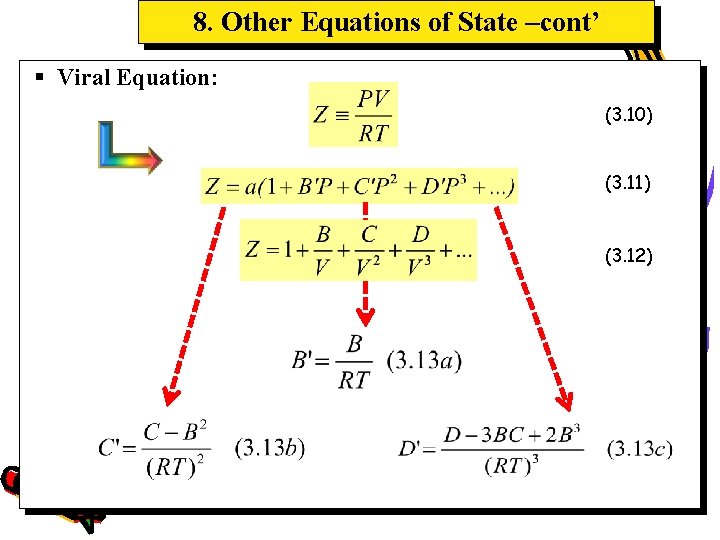
8. Other Equations of State –cont’ § Viral Equation: (3. 10) (3. 11) (3. 12)

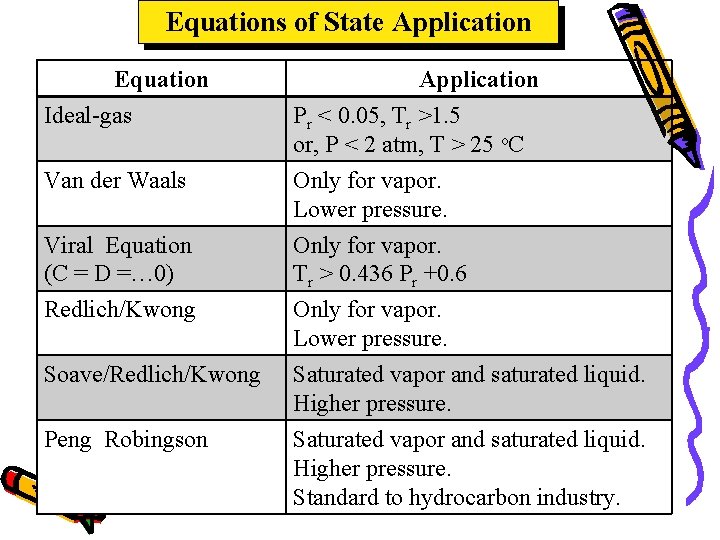
Equations of State Application Equation Ideal-gas Van der Waals Application Pr < 0. 05, Tr >1. 5 or, P < 2 atm, T > 25 o. C Only for vapor. Lower pressure. Viral Equation (C = D =… 0) Only for vapor. Tr > 0. 436 Pr +0. 6 Redlich/Kwong Only for vapor. Lower pressure. Saturated vapor and saturated liquid. Higher pressure. Standard to hydrocarbon industry. Soave/Redlich/Kwong Peng Robingson

Thank you Prepared by, MISS RAHIMAH OTHMAN
 Volumetric properties of pure fluids
Volumetric properties of pure fluids Pt=po(1+r)t
Pt=po(1+r)t Ert controller
Ert controller Ert tool
Ert tool Ert diagram
Ert diagram Ert erp definition
Ert erp definition Ert erp definition
Ert erp definition Ert 1 programa
Ert 1 programa Ert malaysia
Ert malaysia Intermediate beam design
Intermediate beam design Residual properties in thermodynamics
Residual properties in thermodynamics Residual properties in thermodynamics
Residual properties in thermodynamics Advanced thermodynamics
Advanced thermodynamics Extensive and intensive properties of thermodynamics
Extensive and intensive properties of thermodynamics Properties of pure substances thermodynamics
Properties of pure substances thermodynamics Gravimetric analysis problems
Gravimetric analysis problems Types of volumetric analysis
Types of volumetric analysis Volumetric analysis lab
Volumetric analysis lab Titration definition
Titration definition Volumetric efficiency
Volumetric efficiency Gravimetric analysis introduction
Gravimetric analysis introduction Oversquare vs undersquare
Oversquare vs undersquare Graduated cylinder vs volumetric flask
Graduated cylinder vs volumetric flask Common laboratory glassware
Common laboratory glassware Define volumetric analysis
Define volumetric analysis Classification of gravimetric analysis
Classification of gravimetric analysis Volumetric efficiency
Volumetric efficiency Iodine and sodium thiosulfate
Iodine and sodium thiosulfate Florence flask and erlenmeyer flask
Florence flask and erlenmeyer flask Principle of volumetric analysis
Principle of volumetric analysis Frosted band pipette
Frosted band pipette Volumetric vs mohr pipette
Volumetric vs mohr pipette Volumetric thirst
Volumetric thirst Titration picture
Titration picture Ma*va=mb*vb
Ma*va=mb*vb Volumetric
Volumetric Volumetric
Volumetric Volumetric efficiency formula
Volumetric efficiency formula Volumetric efficiency formula
Volumetric efficiency formula Formula sae
Formula sae