Equivalence Point Definition Equivalence Point the stoichiometric end

- Slides: 8

Equivalence Point

Definition Equivalence Point: the stoichiometric end of a neutralization reaction. Example: Na. OH + HCl H 2 O + Na. Cl What is the mole ratio for the entire reaction? The Equivalence Point would be when 1 mol Na. OH and 1 mol HCl produce 1 mol H 2 O and 1 mol Na. Cl.

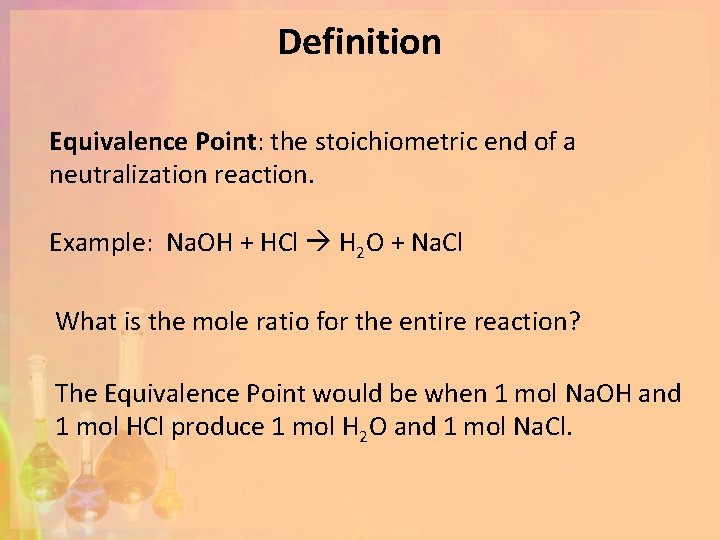
How do we know when we have reached the equivalence point? We use a graph.

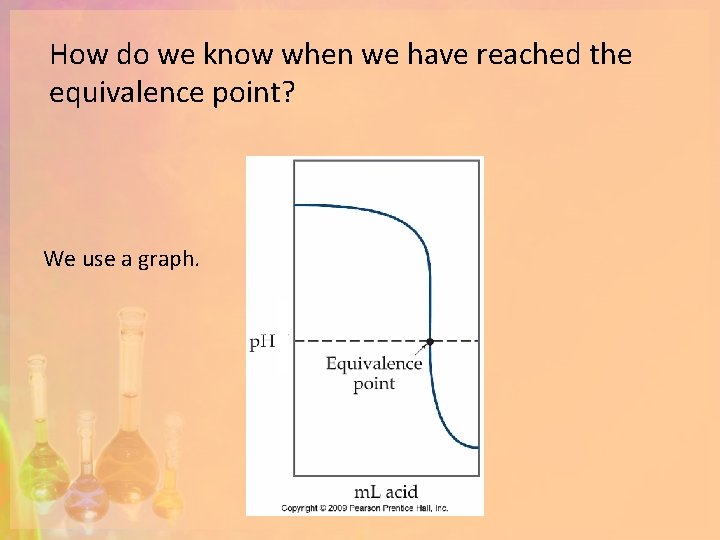
Equivalence Point and ions (HCl + Na. OH) What is the balanced equation for this reaction? Notice the equivalence point. Is the equivalence point the same as the endpoint? HCl + Na. OH Na. Cl + H 2 O Notice the acid is completely dissociated. Notice the equivalence point. What is the mole ratio?

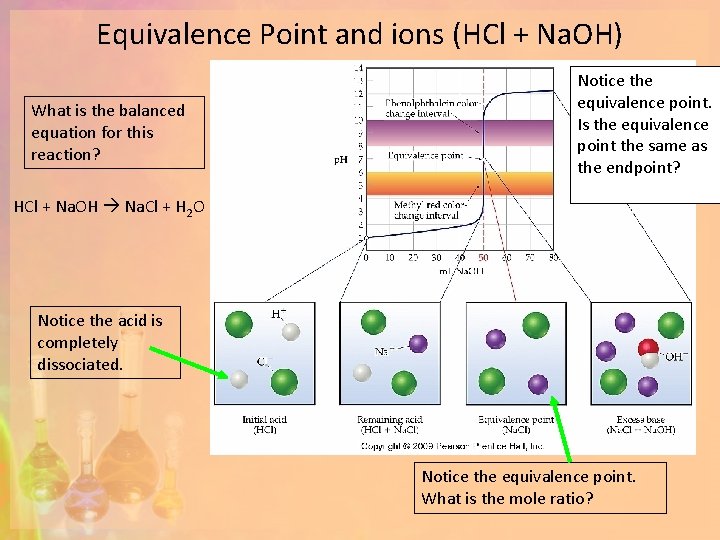
Another Example (CH 3 COOH + Na. OH) What is the balanced equation for this reaction? Notice the equivalence point. Is the equivalence point the same as the endpoint? CH 3 COOH + Na. OH CH 3 COONa + H 2 O Notice the acid is NOT completely dissociated. Notice the equivalence point. What is the mole ratio?

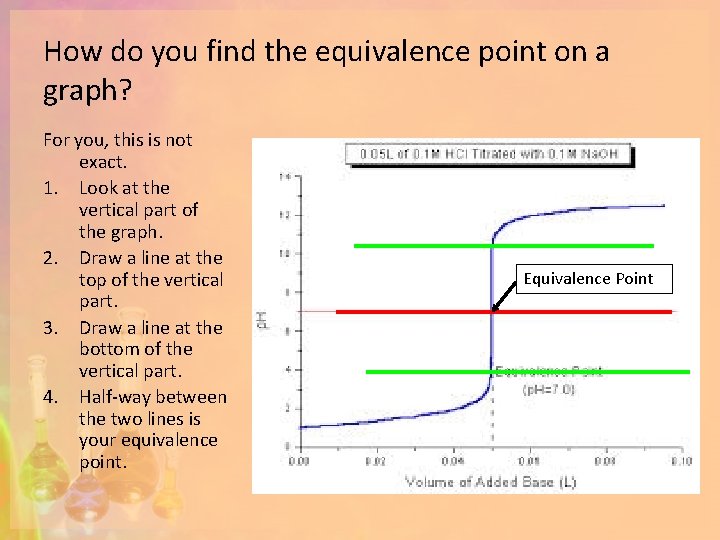
How do you find the equivalence point on a graph? For you, this is not exact. 1. Look at the vertical part of the graph. 2. Draw a line at the top of the vertical part. 3. Draw a line at the bottom of the vertical part. 4. Half-way between the two lines is your equivalence point. Equivalence Point

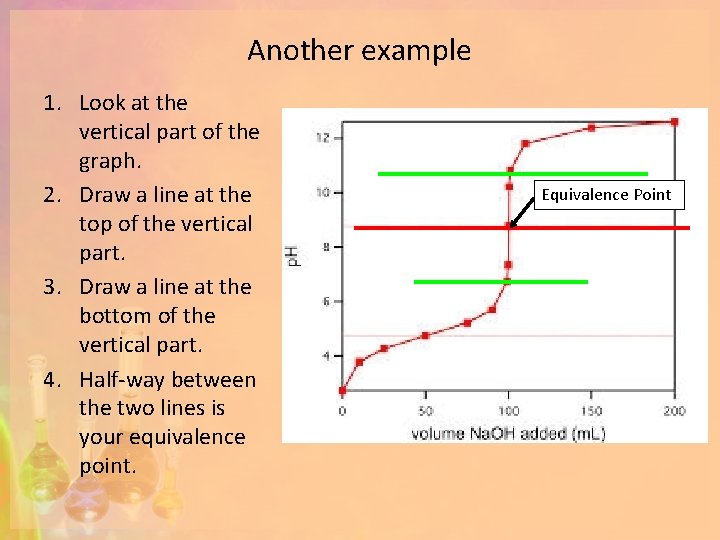
Another example 1. Look at the vertical part of the graph. 2. Draw a line at the top of the vertical part. 3. Draw a line at the bottom of the vertical part. 4. Half-way between the two lines is your equivalence point. Equivalence Point

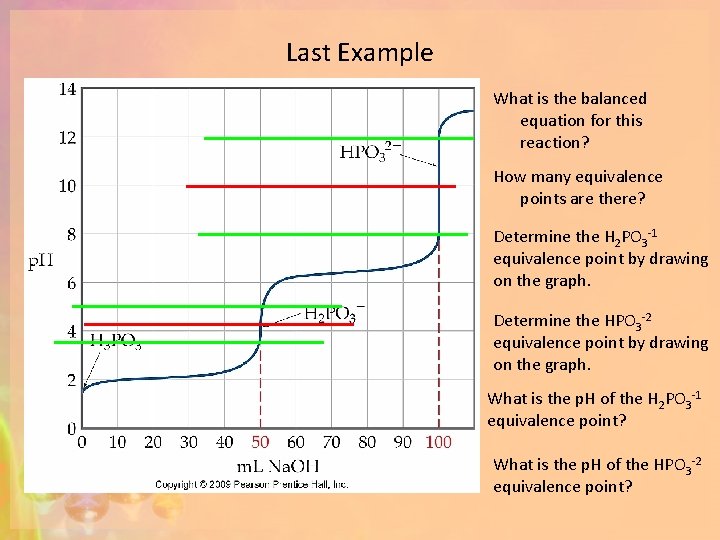
Last Example What is the balanced equation for this reaction? How many equivalence points are there? Determine the H 2 PO 3 -1 equivalence point by drawing on the graph. Determine the HPO 3 -2 equivalence point by drawing on the graph. What is the p. H of the H 2 PO 3 -1 equivalence point? What is the p. H of the HPO 3 -2 equivalence point?