Equilibrium Where vs Why The value of K

Equilibrium – Where? vs. Why? The value of K is an indication of WHERE the equilibrium rests. We haven’t addressed WHY the equilibrium exists where it does. ΔG
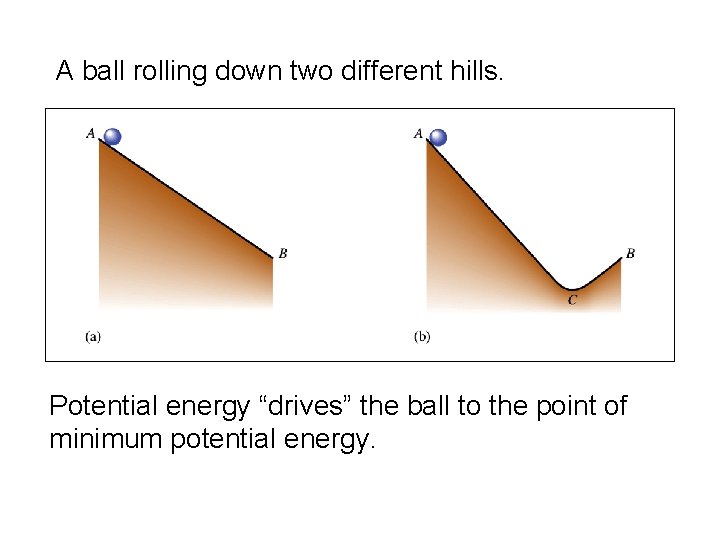
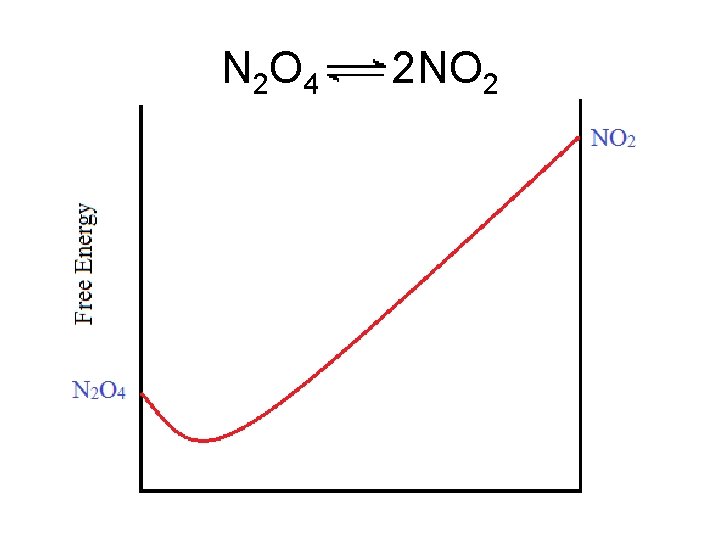
A ball rolling down two different hills. Potential energy “drives” the ball to the point of minimum potential energy.
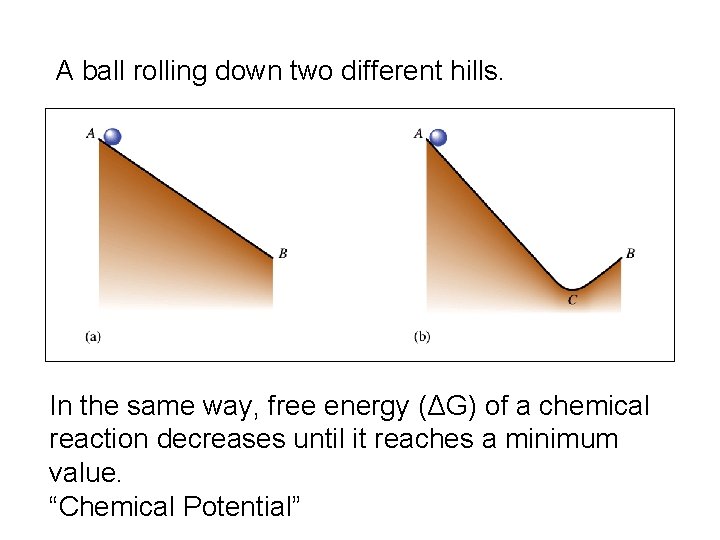
A ball rolling down two different hills. In the same way, free energy (ΔG) of a chemical reaction decreases until it reaches a minimum value. “Chemical Potential”
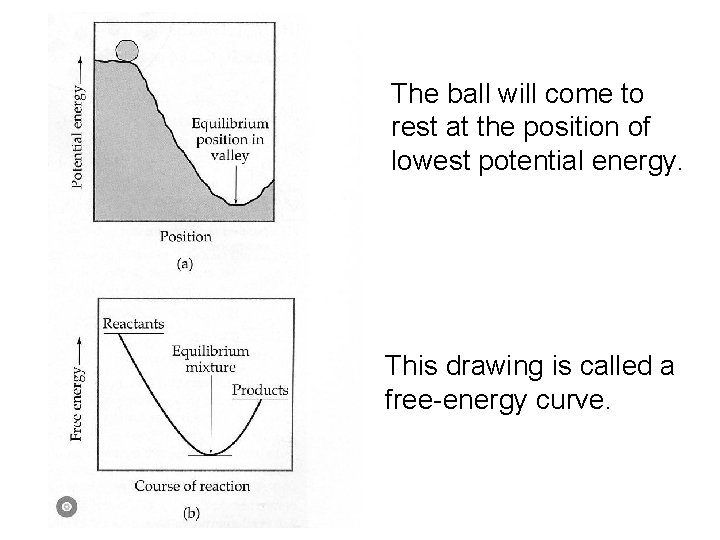
The ball will come to rest at the position of lowest potential energy. A reaction will proceed This drawing is called a to the point where free-energy curve. energy is lowest.
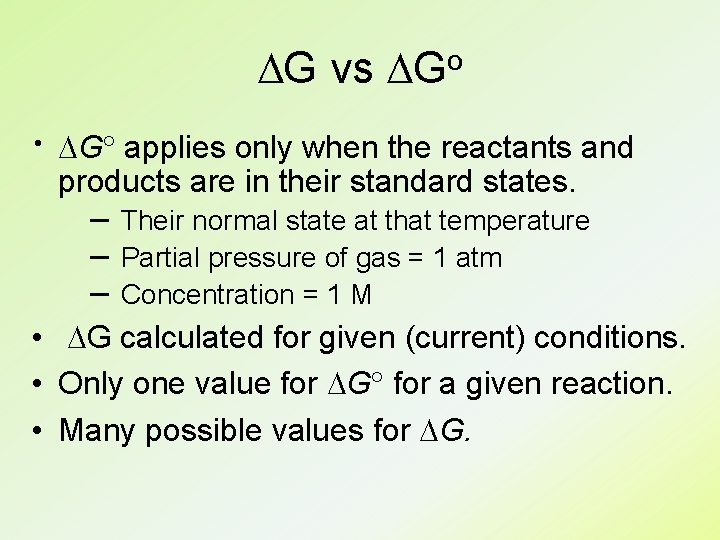
DG vs DGo × DG applies only when the reactants and products are in their standard states. – Their normal state at that temperature – Partial pressure of gas = 1 atm – Concentration = 1 M • DG calculated for given (current) conditions. • Only one value for DG for a given reaction. • Many possible values for DG.
N 2 O 4 2 NO 2
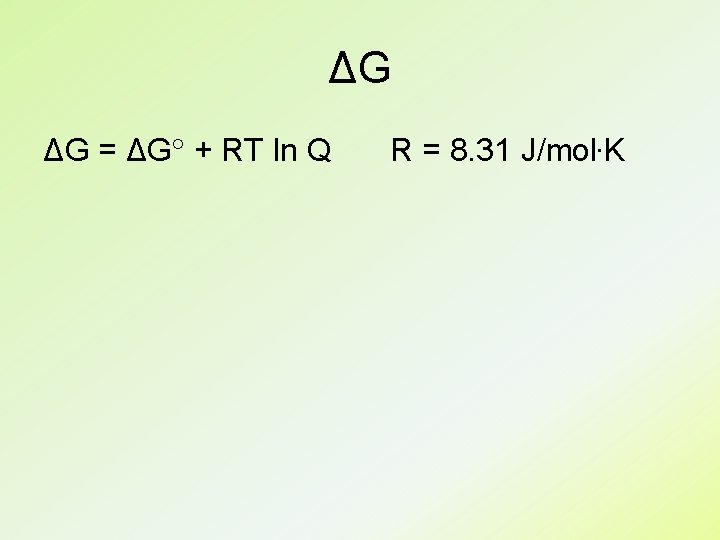
ΔG ΔG = ΔG + RT ln Q R = 8. 31 J/mol. K
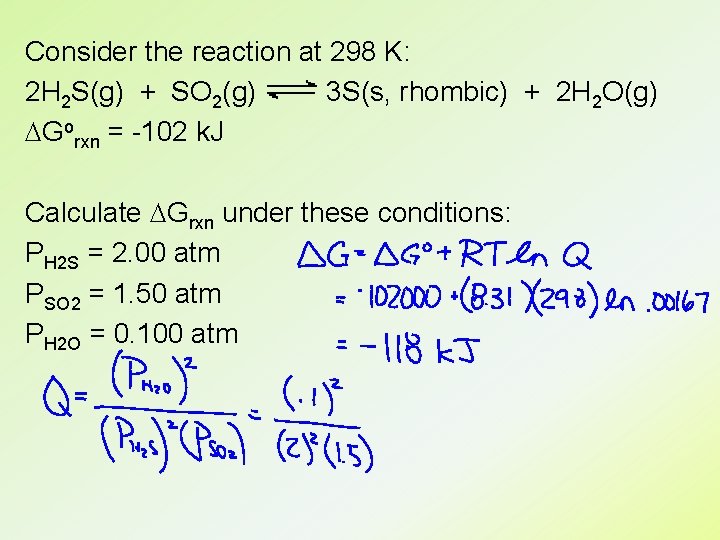
Consider the reaction at 298 K: 2 H 2 S(g) + SO 2(g) 3 S(s, rhombic) + 2 H 2 O(g) DGorxn = -102 k. J Calculate DGrxn under these conditions: PH 2 S = 2. 00 atm PSO 2 = 1. 50 atm PH 2 O = 0. 100 atm

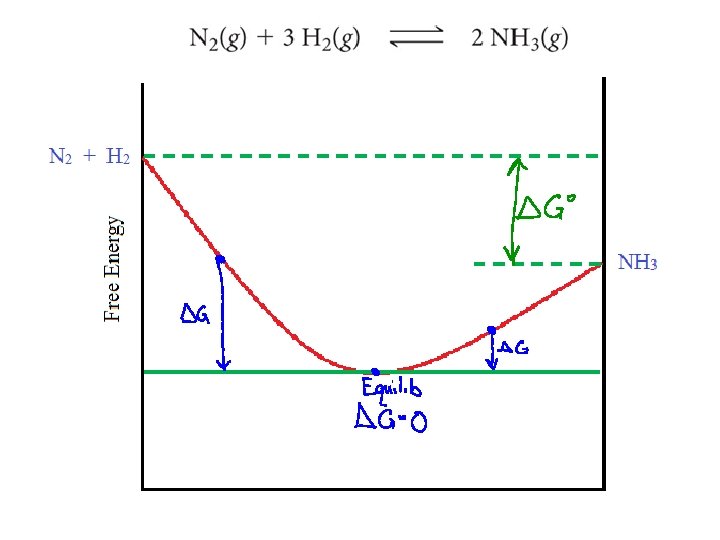
ΔG ΔG = ΔG + RT ln Q At equilibrium, ΔG = 0 and Q = K ΔG = -RT ln K
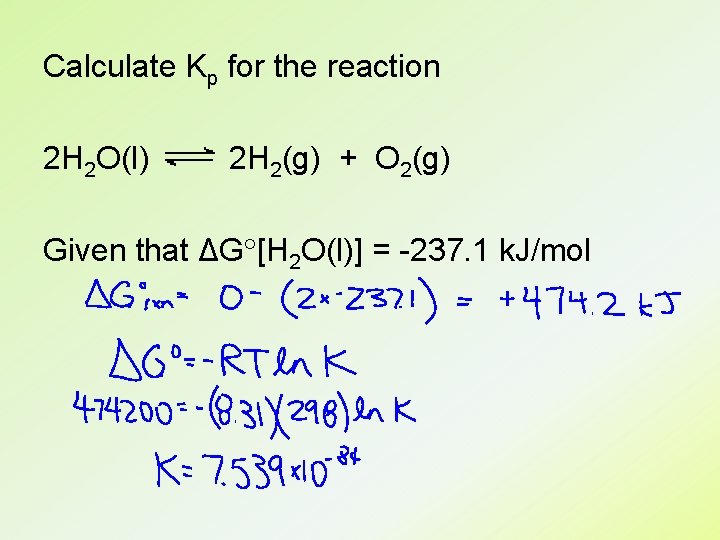
Calculate Kp for the reaction 2 H 2 O(l) 2 H 2(g) + O 2(g) Given that ΔG [H 2 O(l)] = -237. 1 k. J/mol
- Slides: 11