Equilibrium Vocabulary for Unit Reversible Rxn Chemical Equilibrium
Equilibrium Vocabulary for Unit: • Reversible Rxn • Chemical Equilibrium • Law of Chemical Equilibrium • Equilibrium Constant • Homogeneous equilibrium • Heterogeneous equilibrium • Le Chậtelier’s Principle • ICE Charts Objectives: • List the characteristics of chemical equilibrium. • Write equilibrium expressions for systems that are at equilibrium. • Calculate equilibrium constants from concentration data. • Describe how various factors affect chemical equilibrium. • Explain how Le Châtelier’s principle applies to equilibrium systems.

What is equilibrium? Chemical reactions often reach a balancing point, or equilibrium. N 2(g) + 3 H 2(g) ↔ 2 NH 3(g)

What is equilibrium? A reversible reaction is a chemical reaction that can occur in both the forward and reverse directions, such as the formation of ammonia. N 2(g) + 3 H 2(g) ↔ 2 NH 3(g)
What is equilibrium? Chemical equilibrium is a state in which the forward and reverse reactions balance each other because they take place at equal rates. This means it is considered to be “dynamic” Equilibrium is a state of action, not inaction.
Equilibrium Expressions Some chemical systems have little tendency to react, others go to completion. The majority reach a state of equilibrium with some of the reactants unconsumed.
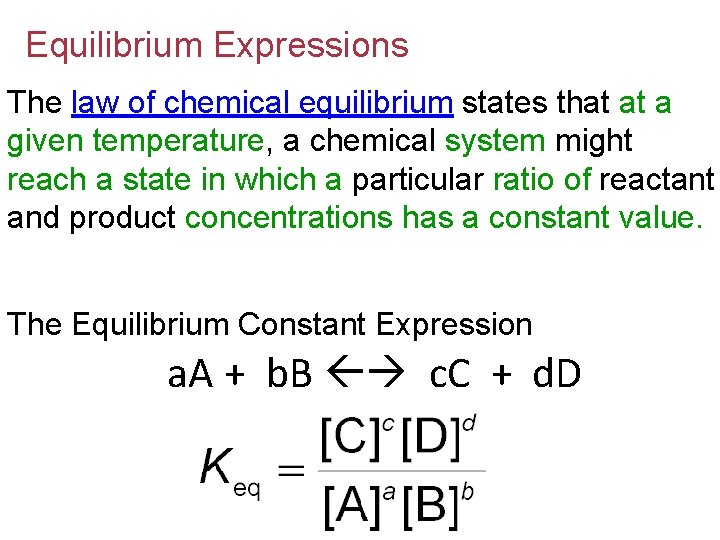
Equilibrium Expressions The law of chemical equilibrium states that at a given temperature, a chemical system might reach a state in which a particular ratio of reactant and product concentrations has a constant value. The Equilibrium Constant Expression a. A + b. B c. C + d. D
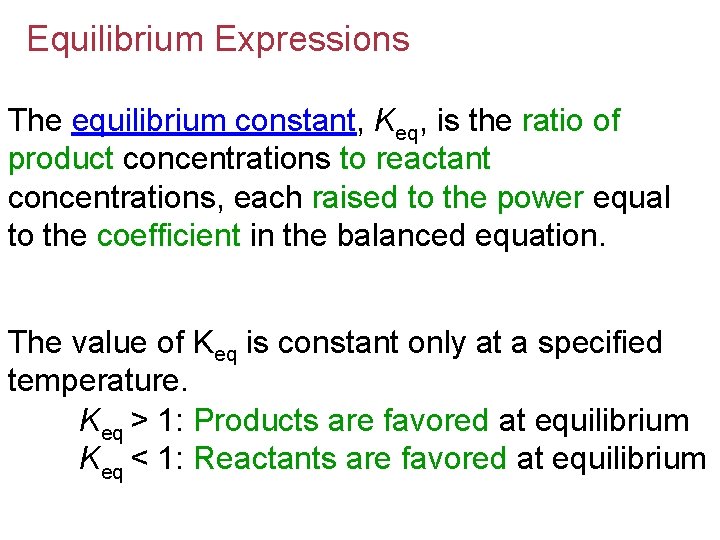
Equilibrium Expressions The equilibrium constant, Keq, is the ratio of product concentrations to reactant concentrations, each raised to the power equal to the coefficient in the balanced equation. The value of Keq is constant only at a specified temperature. Keq > 1: Products are favored at equilibrium Keq < 1: Reactants are favored at equilibrium
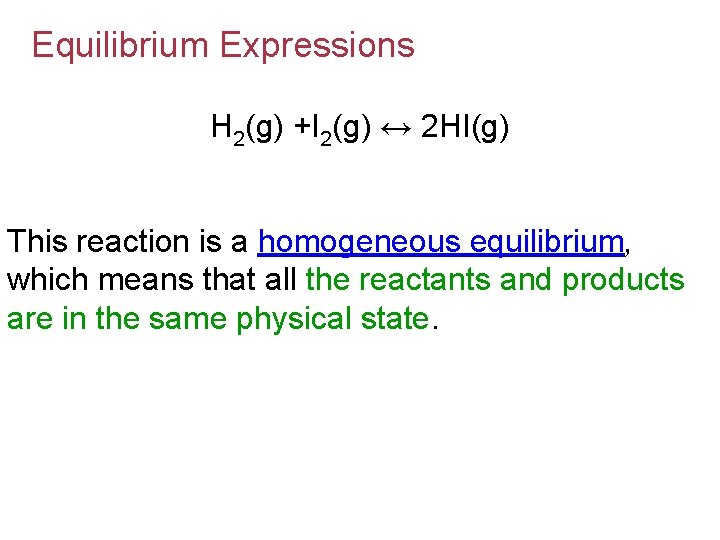
Equilibrium Expressions H 2(g) +I 2(g) ↔ 2 HI(g) This reaction is a homogeneous equilibrium, which means that all the reactants and products are in the same physical state.
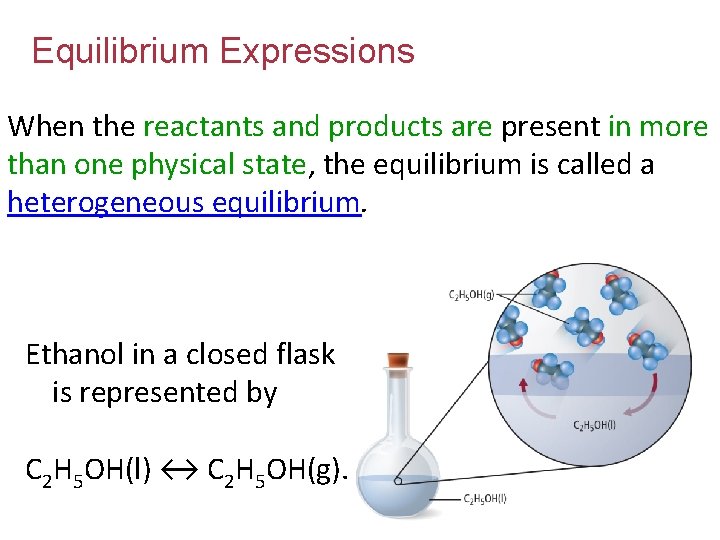
Equilibrium Expressions When the reactants and products are present in more than one physical state, the equilibrium is called a heterogeneous equilibrium. Ethanol in a closed flask is represented by C 2 H 5 OH(l) ↔ C 2 H 5 OH(g).
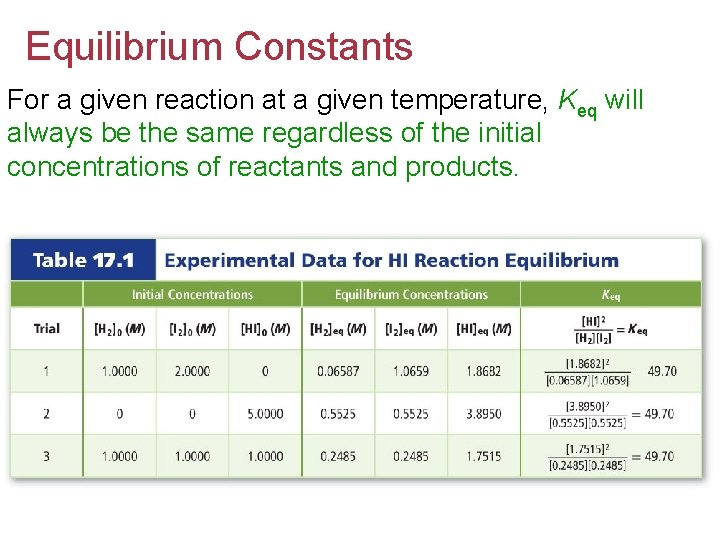
Equilibrium Constants For a given reaction at a given temperature, Keq will always be the same regardless of the initial concentrations of reactants and products.

Writing Keq Only the reactants and products that are aqueous or gaseous are shown in an equilibrium expression. - Pure solids and liquids do not change in concentration, so they will not change the value of Keq and therefore, they do not appear in the equilibrium expression.
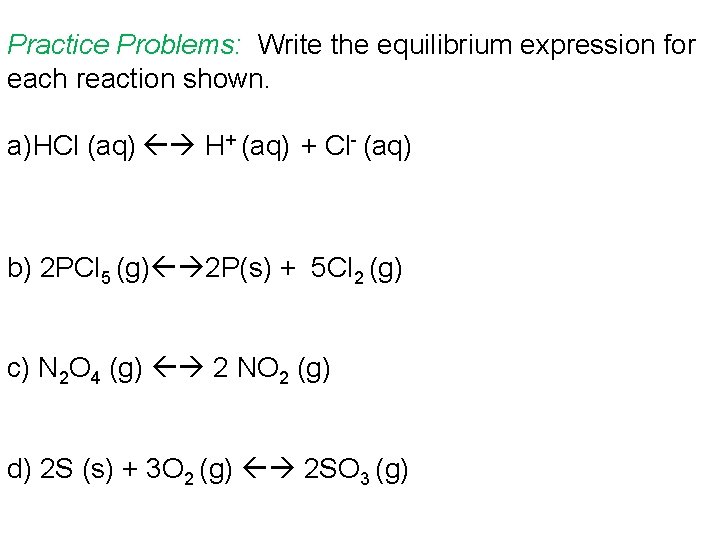
Practice Problems: Write the equilibrium expression for each reaction shown. a)HCl (aq) H+ (aq) + Cl- (aq) b) 2 PCl 5 (g) 2 P(s) + 5 Cl 2 (g) c) N 2 O 4 (g) 2 NO 2 (g) d) 2 S (s) + 3 O 2 (g) 2 SO 3 (g)
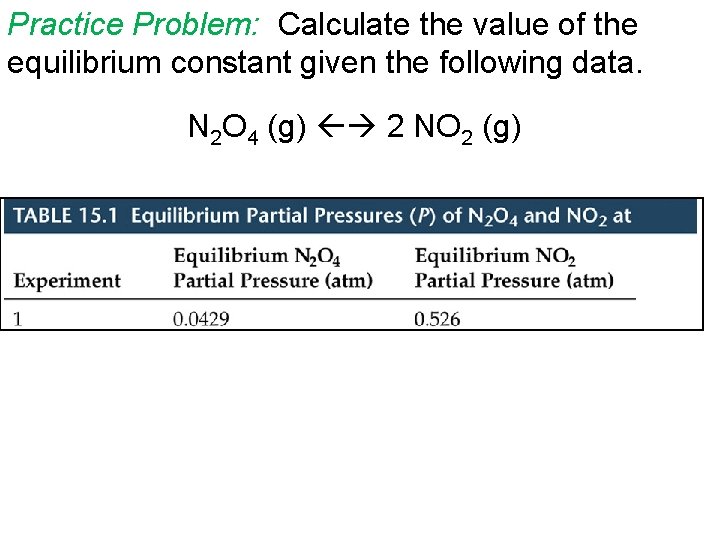
Practice Problem: Calculate the value of the equilibrium constant given the following data. N 2 O 4 (g) 2 NO 2 (g)

How to Calculate Keq Step 1: Make an ICE chart Step 2: Fill in what you know Step 3: Write in the change (Remember that moles are quantitative) Step 4: Multiply the change by the coefficient in the balanced equation. Step 5: Find the equilibrium concentrations of all species. Step 6: Plug into the Keq expression
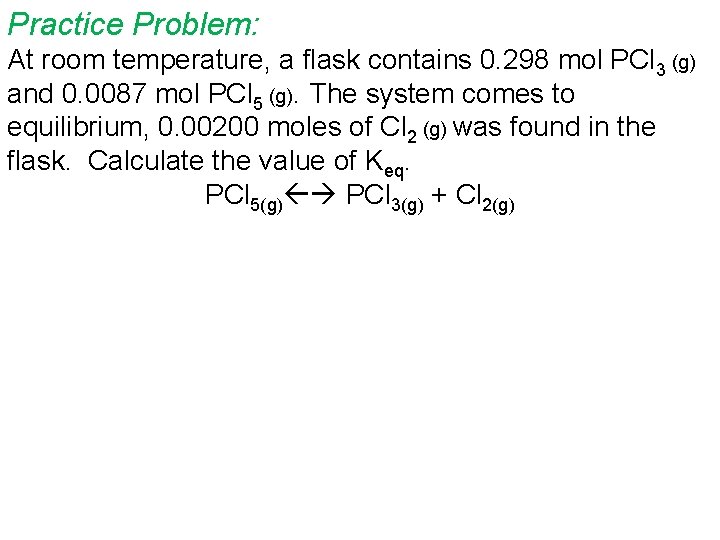
Practice Problem: At room temperature, a flask contains 0. 298 mol PCl 3 (g) and 0. 0087 mol PCl 5 (g). The system comes to equilibrium, 0. 00200 moles of Cl 2 (g) was found in the flask. Calculate the value of Keq. PCl 5(g) PCl 3(g) + Cl 2(g)
![How to Calculate Keq [PCl 5] [PCl 3] + Initial Change Equilibrium 0. 0087 How to Calculate Keq [PCl 5] [PCl 3] + Initial Change Equilibrium 0. 0087](http://slidetodoc.com/presentation_image_h2/08832e697b9e8381609f18a696809043/image-16.jpg)
How to Calculate Keq [PCl 5] [PCl 3] + Initial Change Equilibrium 0. 0087 M − 0. 002 M [Cl 2] 0. 298 M 0 + 0. 002 M 0. 0067 M 0. 300 M 0. 002 M Keq = [ PCl 3][Cl 2] [PCl 5] Keq = [0. 3][0. 002] = 0. 08955≈ 0. 09 [0. 0067] .
![Calculating Equilibrium Concentrations Practice Problem: Calculate the equilibrium concentration of [H+] when the initial Calculating Equilibrium Concentrations Practice Problem: Calculate the equilibrium concentration of [H+] when the initial](http://slidetodoc.com/presentation_image_h2/08832e697b9e8381609f18a696809043/image-17.jpg)
Calculating Equilibrium Concentrations Practice Problem: Calculate the equilibrium concentration of [H+] when the initial concentration of HCN is 0. 3 M. (M = Molarity) HCN(aq) H+(aq) + CN−(aq) Keq = 4. 9 x 10− 10 Since we don’t know how much the “change” will be, we call it “x”. [HCN] Initial Change Equilibrium [H+] + [CN−] 0. 3 M 0 0 −x +x +x 0. 3 − x
![Calculating Equilibrium Concentrations [HCN] Initial Change Equilibrium [H+] + [CN−] 0. 3 M 0 Calculating Equilibrium Concentrations [HCN] Initial Change Equilibrium [H+] + [CN−] 0. 3 M 0](http://slidetodoc.com/presentation_image_h2/08832e697b9e8381609f18a696809043/image-18.jpg)
Calculating Equilibrium Concentrations [HCN] Initial Change Equilibrium [H+] + [CN−] 0. 3 M 0 0 −x +x +x 0. 3 − x If x is small enough to not effect the 0. 3 M, it can be ignored in the (0. 3 - x). If it is not less than 5% of the original concentration you’ll need the quadratic formula

Le Châtelier’s Principle was proposed in 1888 and states that if stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. Stress is any kind of change in a system that upsets the equilibrium.
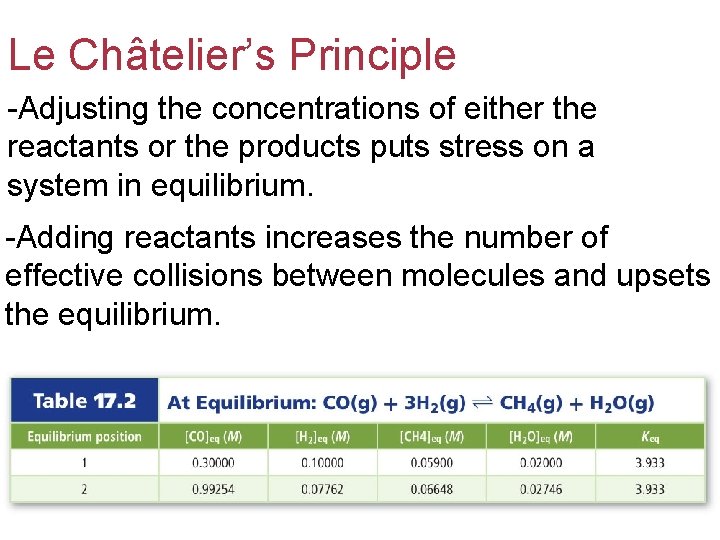
Le Châtelier’s Principle -Adjusting the concentrations of either the reactants or the products puts stress on a system in equilibrium. -Adding reactants increases the number of effective collisions between molecules and upsets the equilibrium.
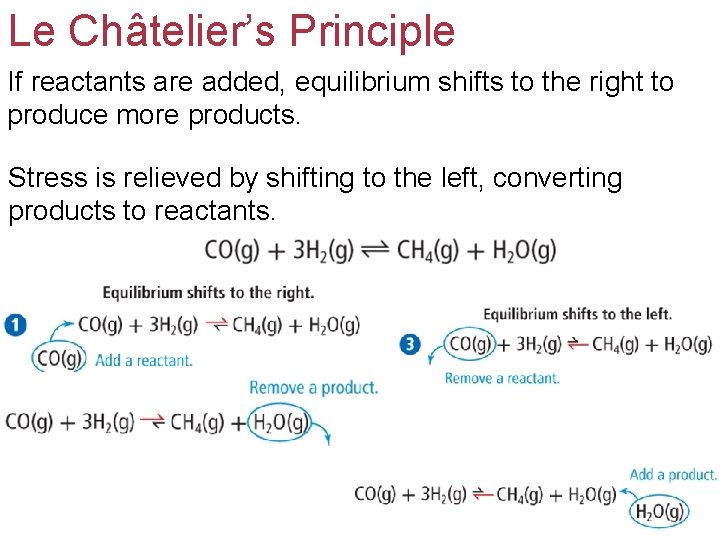
Le Châtelier’s Principle If reactants are added, equilibrium shifts to the right to produce more products. Stress is relieved by shifting to the left, converting products to reactants.
Le Châtelier’s Principle Other stresses that cause a shift in equilibrium: -Pressure when increased will cause the equilibrium to shift towards the side with less molecules. -Volume when decreased, increases the pressure and which will cause a shift to the side with less molecules. -If the number of moles is the same on both sides of the equation, changes in pressure and volume have no effect on the equilibrium.
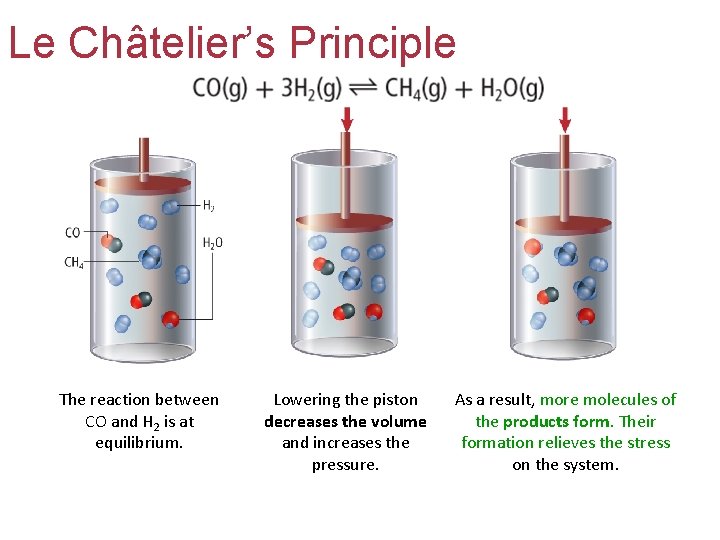
Le Châtelier’s Principle The reaction between CO and H 2 is at equilibrium. Lowering the piston decreases the volume and increases the pressure. As a result, more molecules of the products form. Their formation relieves the stress on the system.
Le Châtelier’s Principle Changes in temperature alter the equilibrium position and the equilibrium constant. If heat is added to an equilibrium system, the equilibrium shifts in the direction in which the heat is used up.
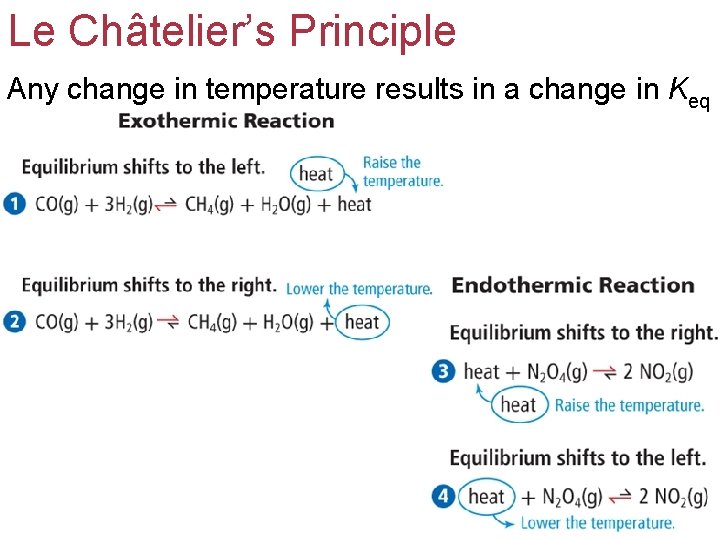
Le Châtelier’s Principle Any change in temperature results in a change in Keq
Le Châtelier’s Principle A catalyzed reaction reaches equilibrium more quickly, but with no change in the amount of product formed.
- Slides: 26