Equilibrium Processes in Gases Equilibrium process Quasistatic process
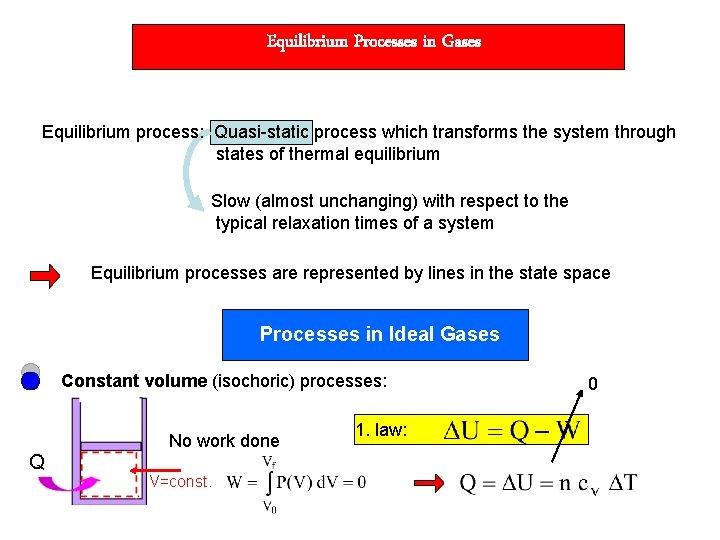
Equilibrium Processes in Gases Equilibrium process: Quasi-static process which transforms the system through states of thermal equilibrium Slow (almost unchanging) with respect to the typical relaxation times of a system Equilibrium processes are represented by lines in the state space Processes in Ideal Gases Constant volume (isochoric) processes: Q No work done V=const. 1. law: 0
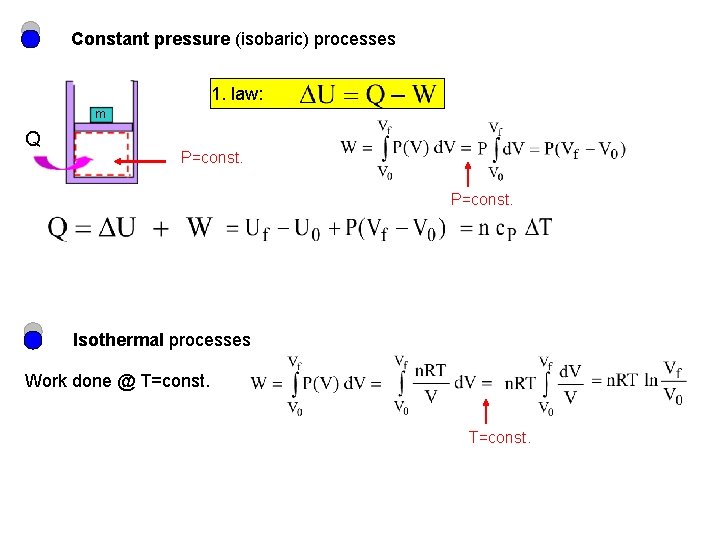
Constant pressure (isobaric) processes 1. law: m Q P=const. Isothermal processes Work done @ T=const.
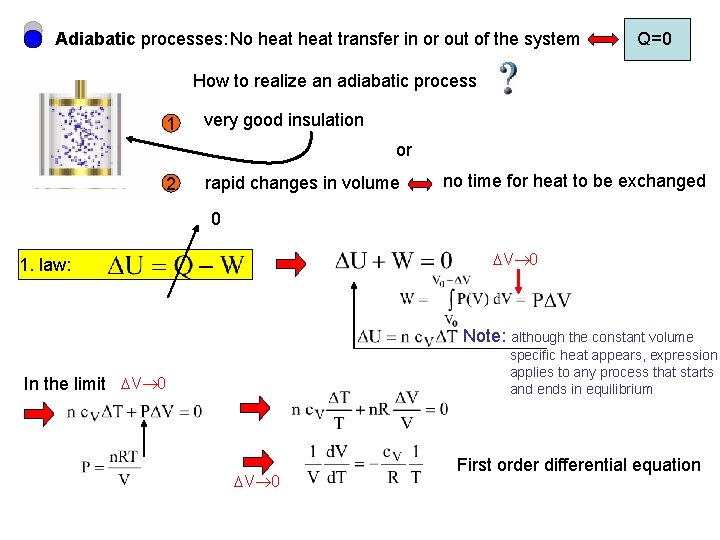
Adiabatic processes: No heat transfer in or out of the system Q=0 How to realize an adiabatic process 1 very good insulation or 2 rapid changes in volume no time for heat to be exchanged 0 V 0 1. law: Note: although the constant volume specific heat appears, expression applies to any process that starts and ends in equilibrium In the limit V 0 First order differential equation
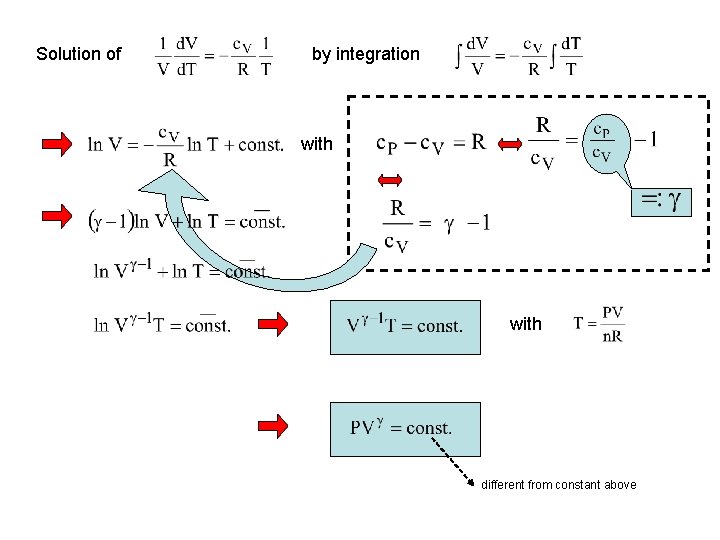
Solution of by integration with different from constant above
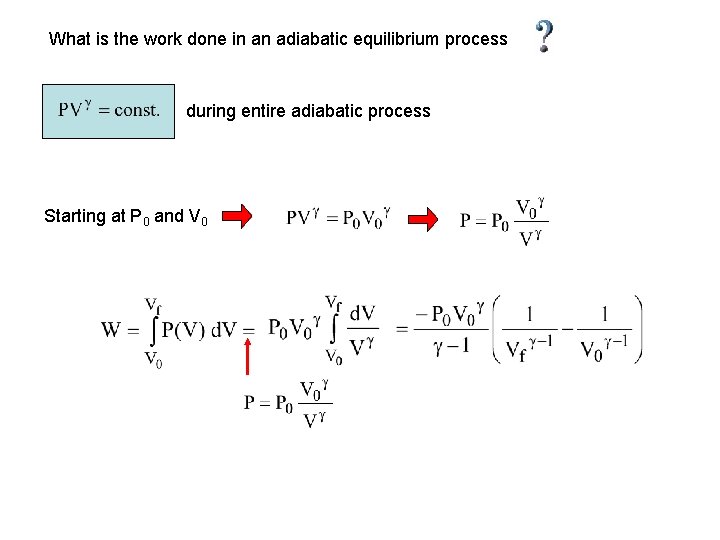
What is the work done in an adiabatic equilibrium process during entire adiabatic process Starting at P 0 and V 0
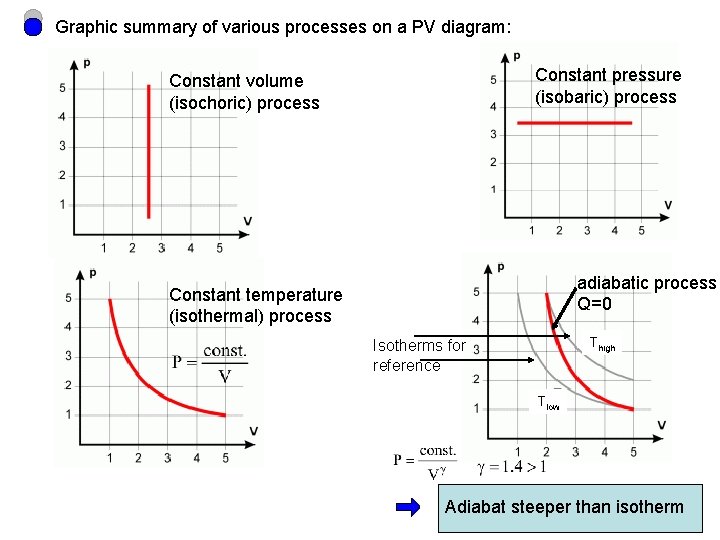
Graphic summary of various processes on a PV diagram: Constant pressure (isobaric) process Constant volume (isochoric) process adiabatic process Q=0 Constant temperature (isothermal) process Thigh Isotherms for reference Tlow Adiabat steeper than isotherm
- Slides: 6