Equilibrium Objectives 1 Understand equilibrium is dynamic two
Equilibrium Objectives: 1. Understand equilibrium is dynamic (two opposite procedure at the same rate at certain temperature); 2. Master the equilibrium calculation (the equilibrium constant and solubility product); 3. Learn the Le Chatelier’s Principle (predict the direction shift when an equilibrium is disturbed).

Concept: How can a chemical reaction happen? The molecules of reactant(s) must collide with enough energy to break the bonds and form new bonds.

Concept: Activation energy (Ea) • The least energy needed for the reaction to occur.

Concept: catalyst • A substance speeds up the reaction and keep itself unchanged in the reaction.
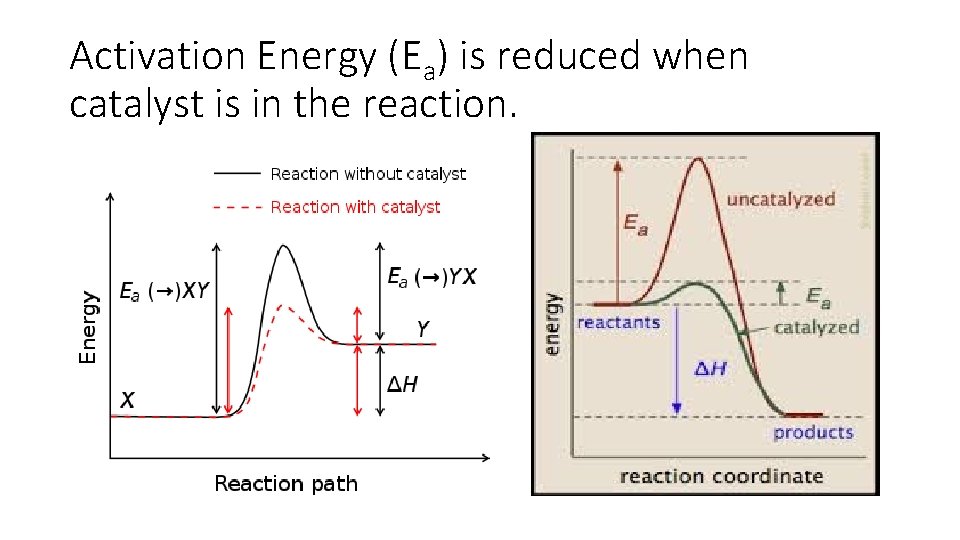
Activation Energy (Ea) is reduced when catalyst is in the reaction.

Concept: equilibrium • The two opposite reactions are at the same rate. It is a dynamic state where the concentrations of all reactants and productions remain the same.

Concept: Equilibrium Expression
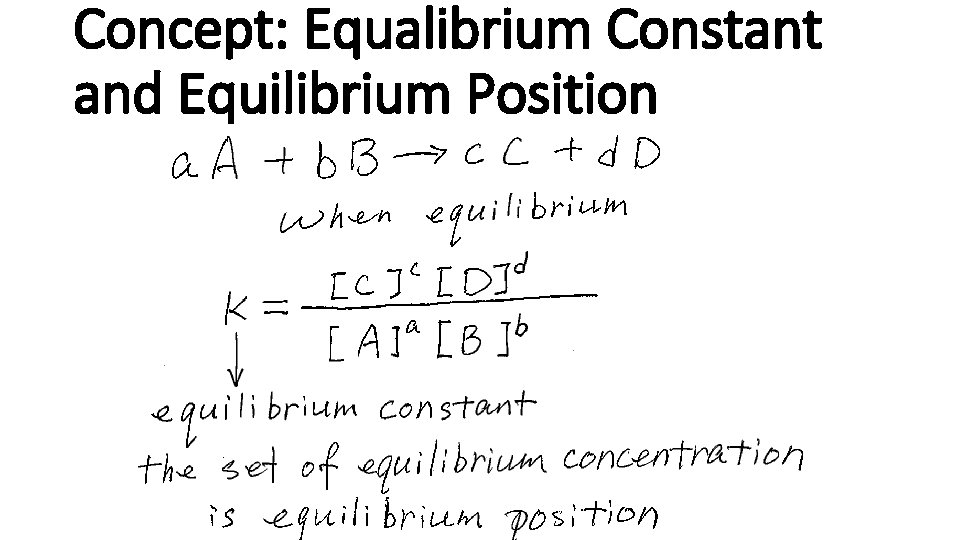
Concept: Equalibrium Constant and Equilibrium Position
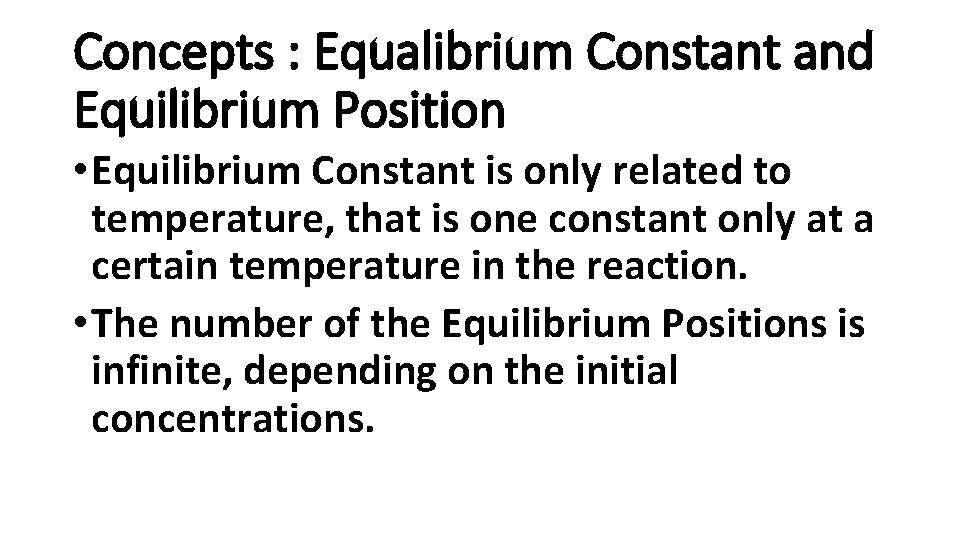
Concepts : Equalibrium Constant and Equilibrium Position • Equilibrium Constant is only related to temperature, that is one constant only at a certain temperature in the reaction. • The number of the Equilibrium Positions is infinite, depending on the initial concentrations.

Heterogeneous Equilibria • Take the concentration of solid and liquid as 1 for calculating equilibrium constant.

Concept: Solubility Equilibria • Ksp, the solubility product, is the equilibrium constant for the slightly dissolving salt.

Concept: the Le Chatlier’s Principle • If a chemical system at equilibrium is stressed, the system will adjust to reduce the stress.

Concept: the Le Chatlier’s Principle • (1) changing the concentration of one of the components of the reaction • (2) changing the pressure on the system • (3) changing the temperature at which the reaction is run.
- Slides: 13