Equilibrium Le Chteliers Principle Chemistry Unit 12 Reaction

Equilibrium & Le. Châtelier’s Principle Chemistry Unit 12: Reaction Rates & Equilibrium Lecture #2

Objectives • Describe what chemical equilibrium IS, and what it ISN’T • Explain Le. Châtelier’s Principle • Describe the effects different stresses have on a system in equilibrium
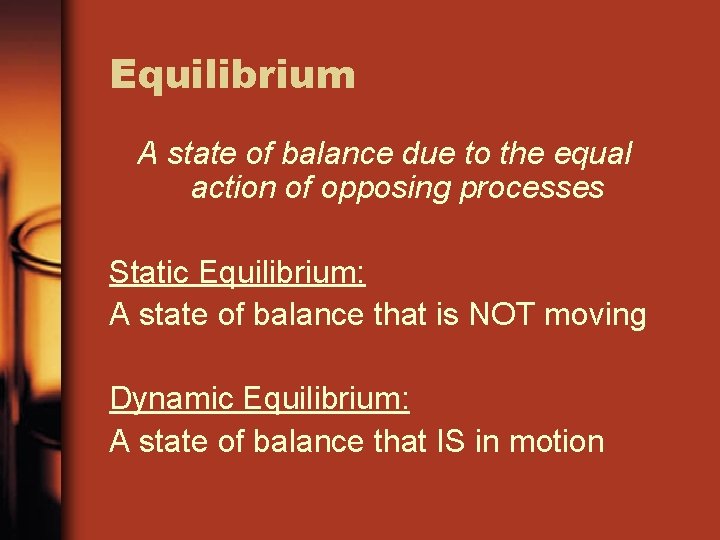
Equilibrium A state of balance due to the equal action of opposing processes Static Equilibrium: A state of balance that is NOT moving Dynamic Equilibrium: A state of balance that IS in motion

Chemical Equilibrium A state of dynamic equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction Reactants are becoming products just as quickly as products are turning back into reactants

What Chemical Equilibrium IS…and what it IS NOT What it IS… When the rate of the forward reaction equals the rate of the reverse reaction What it IS NOT… The reaction does NOT stop. The forward and reverse are just happening at the same rate, so concentrations stop changing Not (necessarily) when the amount of reactants equals the amount of products
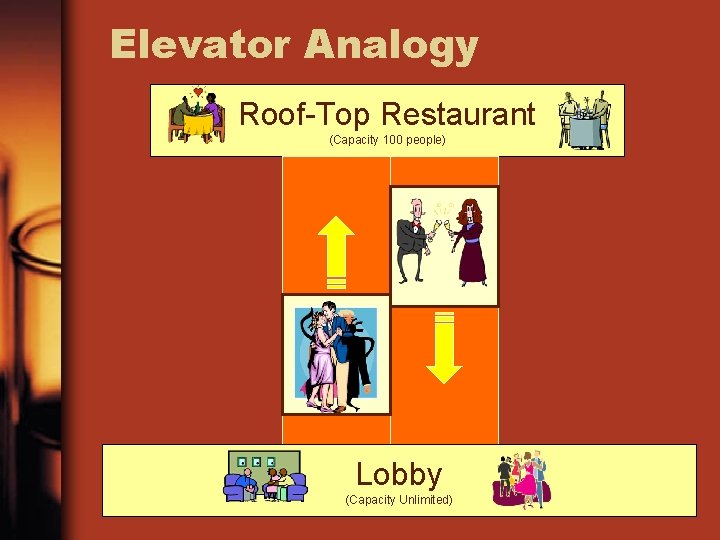
Elevator Analogy Roof-Top Restaurant (Capacity 100 people) Lobby (Capacity Unlimited)

Equilibrium Particulars • Only happens for reversible reactions • It is not reached immediately • The quantity of reactants and products does NOT have to be the same when equilibrium is reached • The rate of exchange (reactants to products and products to reactants) is the same once equilibrium is reached.

Le Châtelier’s Principle • When a stress is applied to a system in equilibrium, the equilibrium will shift to relieve the stress. • This means…either the forward or reverse reaction, is favored (will speed up) until a new equilibrium point is reached

Possible Stressors • Concentration • Temperature • Pressure

Concentration Increasing the concentration of a substance causes more collisions on that side of the equation, and the equilibrium will shift to the other side H 2 CO 3(aq) CO 2(g) + H 2 O(l) Increasing the concentration of the reactants… Equilibrium shifts toward the products The products are favored

Temperature (put heat into the equation first) Adding heat causes more collisions on that side of the equation, so equilibrium shifts to the other side 2 SO 2(g) + O 2(g) 2 SO 3(g) + heat If the temperature is increased… Equilibrium shifts toward the reactants If the temperature is decreased… Equilibrium shifts toward the products

Pressure (determine the number of moles on each side first) • Only affects gases • Increasing the pressure shifts the equilibrium to the side with fewer moles of gas • If the # of moles are equal on both sides…no shift in equilibrium.

Pressure H 2 CO 3(aq) CO 2(g) + H 2 O(l) 0 mol of (g) on left… 1 mol of (g) on right Increasing the pressure… Decreasing the pressure (like leaving the cap off the bottle of soda)…

Pressure 2 SO 2(g) + O 2(g) 2 SO 3(g) + heat mol (g) on left… mol (g) on right Increase in pressure shifts equilibrium to the Decrease in pressure shifts equilibrium to the
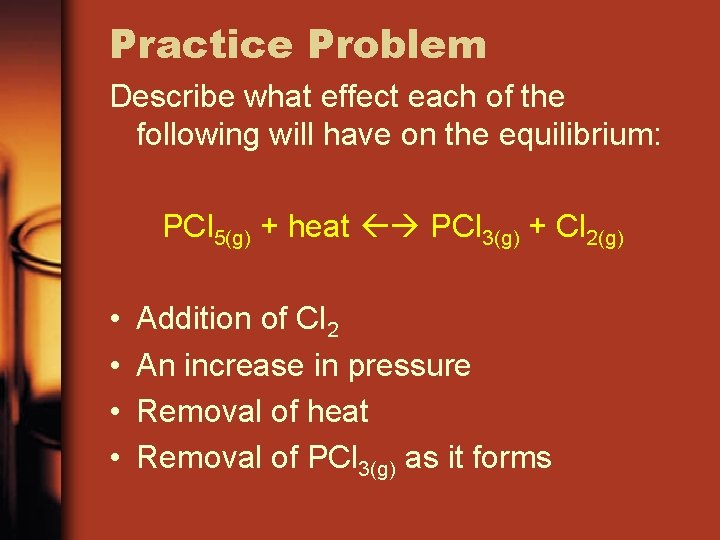
Practice Problem Describe what effect each of the following will have on the equilibrium: PCl 5(g) + heat PCl 3(g) + Cl 2(g) • • Addition of Cl 2 An increase in pressure Removal of heat Removal of PCl 3(g) as it forms

Write Summary & Questions
- Slides: 16