Equilibrium Law Introduction to the Equilibrium law Read

Equilibrium Law
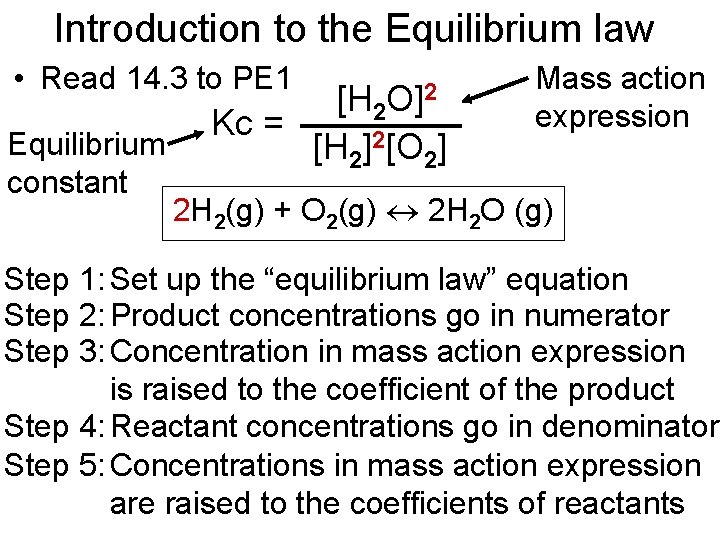
Introduction to the Equilibrium law • Read 14. 3 to PE 1 Equilibrium constant O]2 [H 2 Kc = [H 2]2[O 2] Mass action expression 2 H 2(g) + O 2(g) 2 H 2 O (g) Step 1: Set up the “equilibrium law” equation Step 2: Product concentrations go in numerator Step 3: Concentration in mass action expression is raised to the coefficient of the product Step 4: Reactant concentrations go in denominator Step 5: Concentrations in mass action expression are raised to the coefficients of reactants

Equilibrium law: important points • State (g, l, s, aq) may or may not be added at this point since we will only be dealing with gasses for this section. Later it will matter. • The equilibrium law includes concentrations of products and reactants in mol/L (M) • The value of Kc will depend on temperature, thus this is listed along with the Kc value • Tabulated values of Kc are unitless • By substituting equilibrium concentrations into equilibrium law, we can calculate Kc … • Do RE 14. 31, 35, 36, 37 (pg. 589)
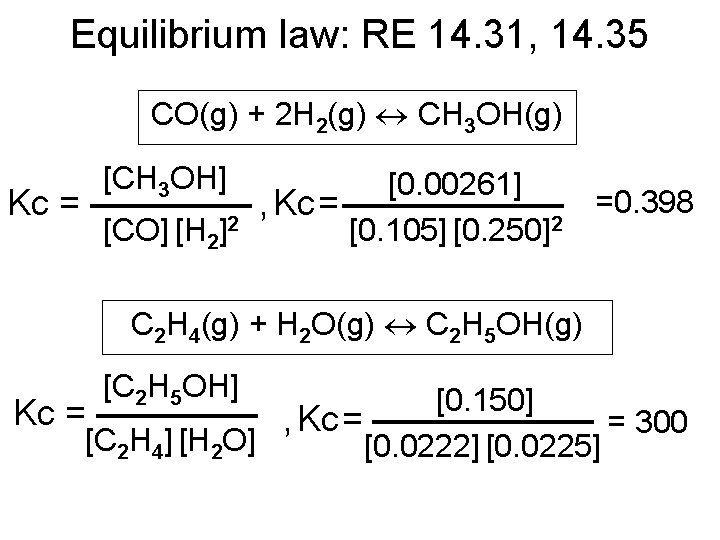
Equilibrium law: RE 14. 31, 14. 35 CO(g) + 2 H 2(g) CH 3 OH(g) [CH 3 OH] [0. 00261] Kc = , Kc = [CO] [H 2]2 [0. 105] [0. 250]2 =0. 398 C 2 H 4(g) + H 2 O(g) C 2 H 5 OH(g) [C 2 H 5 OH] [0. 150] Kc = , Kc = = 300 [C 2 H 4] [H 2 O] [0. 0222] [0. 0225]
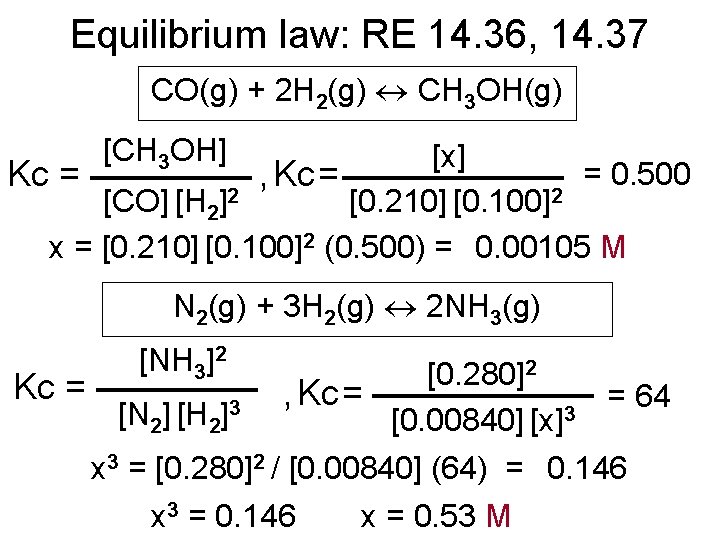
Equilibrium law: RE 14. 36, 14. 37 CO(g) + 2 H 2(g) CH 3 OH(g) [CH 3 OH] [x] = 0. 500 Kc = , Kc = [CO] [H 2]2 [0. 210] [0. 100]2 x = [0. 210] [0. 100]2 (0. 500) = 0. 00105 M N 2(g) + 3 H 2(g) 2 NH 3(g) [NH 3]2 2 [0. 280] Kc = , Kc = = 64 3 [N 2] [H 2] [0. 00840] [x]3 x 3 = [0. 280]2 / [0. 00840] (64) = 0. 146 x 3 = 0. 146 x = 0. 53 M
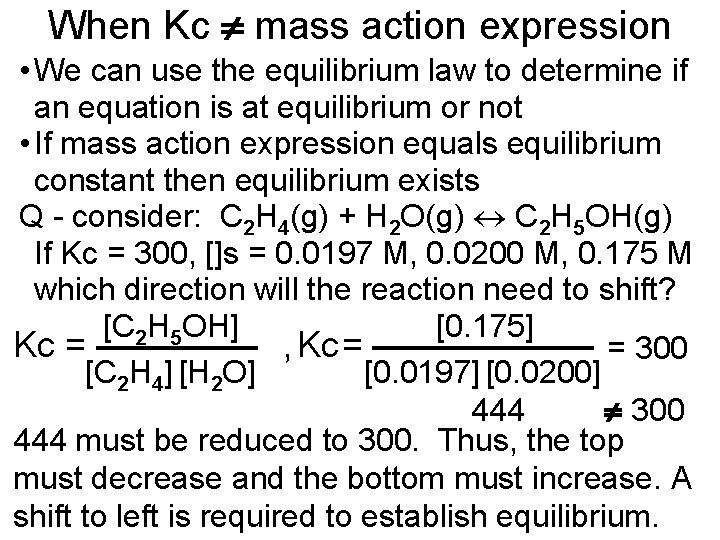
When Kc mass action expression • We can use the equilibrium law to determine if an equation is at equilibrium or not • If mass action expression equals equilibrium constant then equilibrium exists Q - consider: C 2 H 4(g) + H 2 O(g) C 2 H 5 OH(g) If Kc = 300, []s = 0. 0197 M, 0. 0200 M, 0. 175 M which direction will the reaction need to shift? [C 2 H 5 OH] [0. 175] Kc = , Kc = = 300 [C 2 H 4] [H 2 O] [0. 0197] [0. 0200] 444 300 444 must be reduced to 300. Thus, the top must decrease and the bottom must increase. A shift to left is required to establish equilibrium.
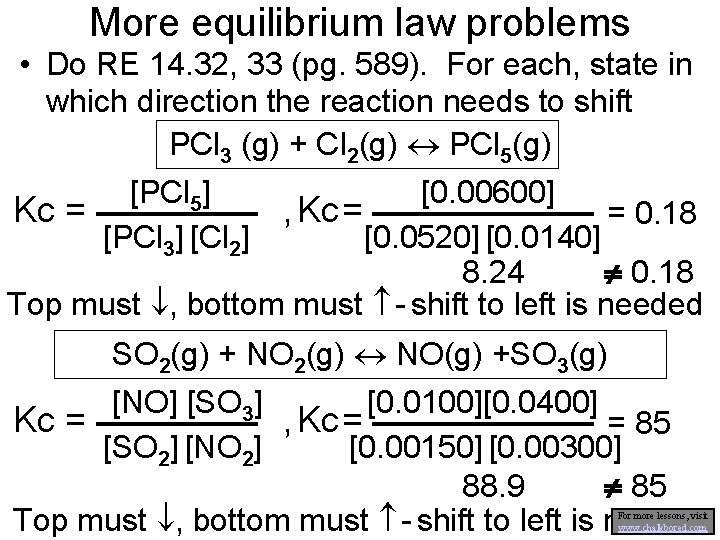
More equilibrium law problems • Do RE 14. 32, 33 (pg. 589). For each, state in which direction the reaction needs to shift PCl 3 (g) + Cl 2(g) PCl 5(g) [PCl 5] [0. 00600] Kc = , Kc = = 0. 18 [PCl 3] [Cl 2] [0. 0520] [0. 0140] 8. 24 0. 18 Top must , bottom must - shift to left is needed SO 2(g) + NO 2(g) NO(g) +SO 3(g) [0. 0100][0. 0400] [NO] [SO 3] Kc = , Kc = = 85 [SO 2] [NO 2] [0. 00150] [0. 00300] 88. 9 85 Top must , bottom must - shift to left is needed For more lessons, visit www. chalkbored. com
- Slides: 7