Equilibrium Honors Chemistry Collision Model How do chemical

Equilibrium Honors Chemistry
Collision Model � How do chemical reactions occur? ◦ Collisions of high energy molecules ◦ If they have: �Correct orientation �Minimum Combined energy ◦ Even when everything is right, some proportion of collisions will return to original state
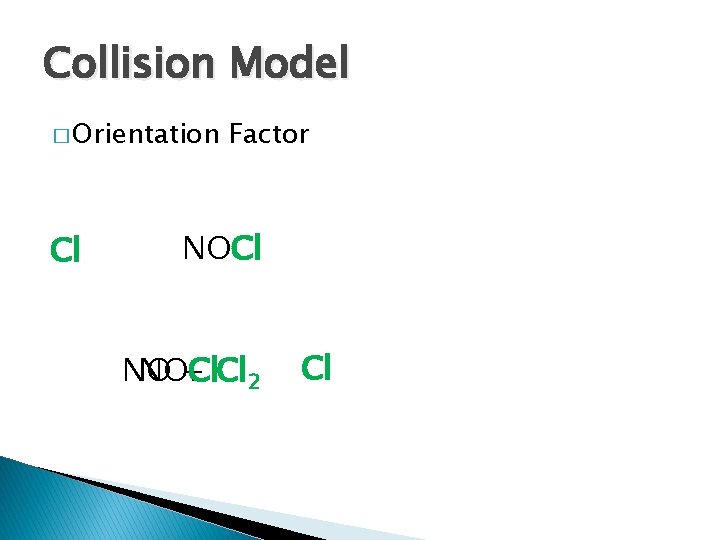
Collision Model � Orientation Cl Factor NOCl NO NOCl + Cl 2 Cl
Energy Factor � Reactants form products ◦ Reactant bonds break �requires energy ◦ Products bonds form �releases energy � Activation Energy (EA) - Minimum Energy required to initiate a chemical reaction ◦ Produces transition state (or activated complex) � ΔE of rxn (or ΔH) has no effect on rate
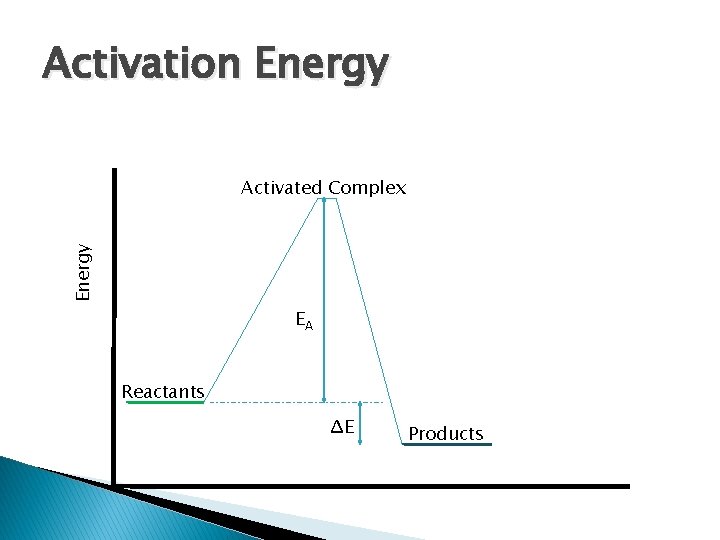
Activation Energy Activated Complex EA Reactants ΔE Products
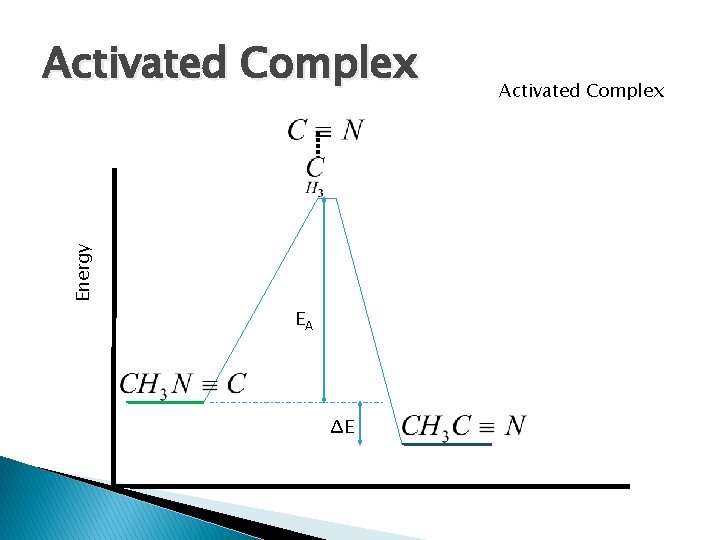
Energy Activated Complex EA ΔE Activated Complex
Reaction Rates � Reaction Rate – speed at which a reaction takes place � Factors ◦ Need Collisions ◦ Need Energy ◦ Need Orientation � What is the effect of Temperature? � What is the effect of Concentration? � What is the effect of Surface Area of a Solid?
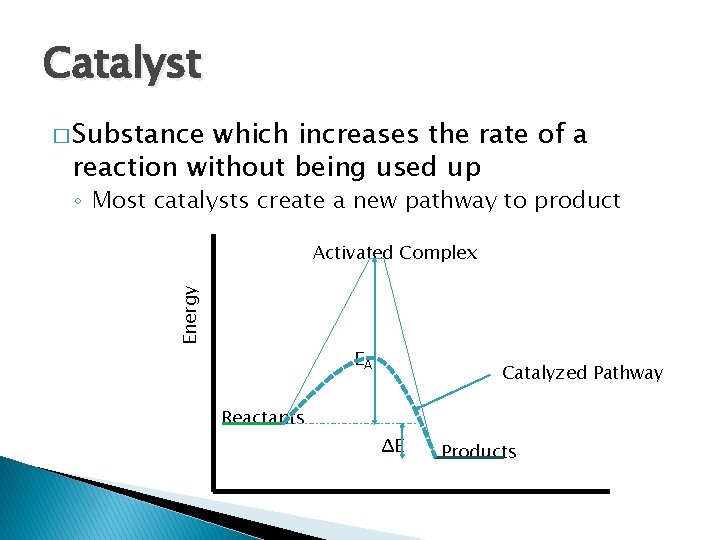
Catalyst � Substance which increases the rate of a reaction without being used up ◦ Most catalysts create a new pathway to product Energy Activated Complex EA Catalyzed Pathway Reactants ΔE Products
Catalyst � Key to a catalyst is that it regenerates � Heterogeneous Catalyst ◦ Catalyst is out of phase with reactants ◦ Catalytic Converter Catalyst ◦ Catalyst is in phase with reactants ◦ Acid in a protein solution � Enzymes O= ◦ Biological Catalysts ◦ Dramatically increase the rate of reaction ◦ Many work by improving orientation of collision O H H � Homogeneous C= O
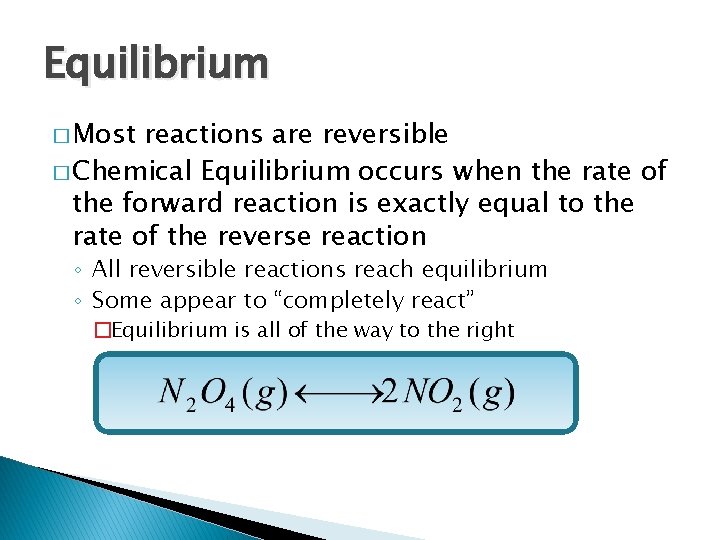
Equilibrium � Most reactions are reversible � Chemical Equilibrium occurs when the rate of the forward reaction is exactly equal to the rate of the reverse reaction ◦ All reversible reactions reach equilibrium ◦ Some appear to “completely react” �Equilibrium is all of the way to the right
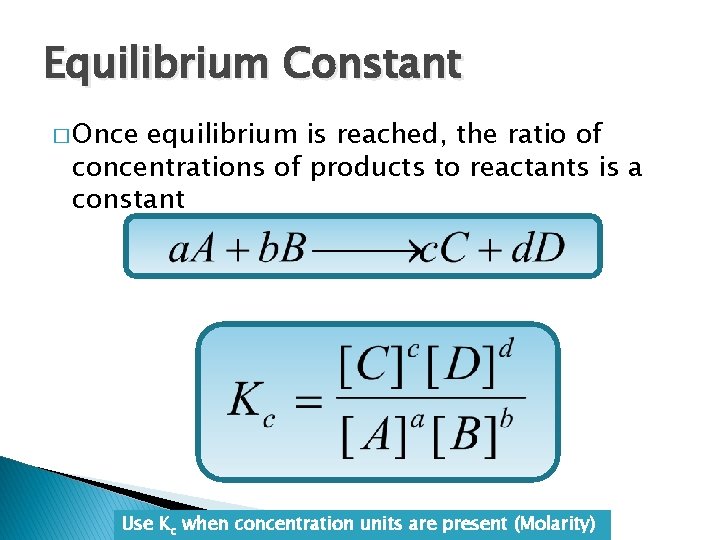
Equilibrium Constant � Once equilibrium is reached, the ratio of concentrations of products to reactants is a constant Use Kc when concentration units are present (Molarity)
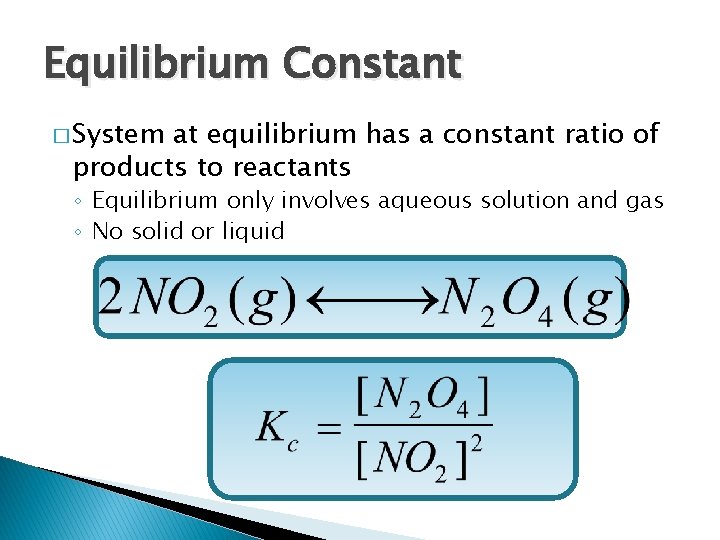
Equilibrium Constant � System at equilibrium has a constant ratio of products to reactants ◦ Equilibrium only involves aqueous solution and gas ◦ No solid or liquid
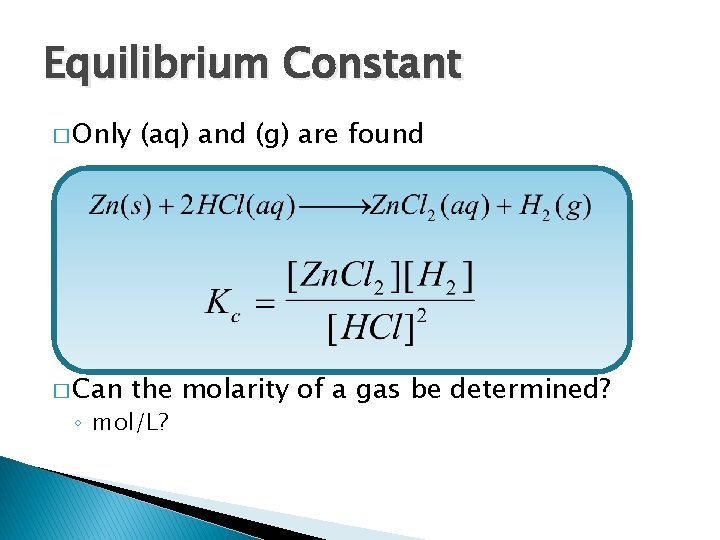
Equilibrium Constant � Only � Can (aq) and (g) are found the molarity of a gas be determined? ◦ mol/L?
![Equilibrium Constant � Calculate K At Equilibrium: [SO 2]=1. 50 [O 2]=1. 25 [SO Equilibrium Constant � Calculate K At Equilibrium: [SO 2]=1. 50 [O 2]=1. 25 [SO](http://slidetodoc.com/presentation_image_h/297fe1fbf49533b279eb72c22fbe1cd3/image-14.jpg)
Equilibrium Constant � Calculate K At Equilibrium: [SO 2]=1. 50 [O 2]=1. 25 [SO 3]=3. 50
![Equilibrium Constant � What is the [O 2] when [SO 3]=4. 85 and [SO Equilibrium Constant � What is the [O 2] when [SO 3]=4. 85 and [SO](http://slidetodoc.com/presentation_image_h/297fe1fbf49533b279eb72c22fbe1cd3/image-15.jpg)
Equilibrium Constant � What is the [O 2] when [SO 3]=4. 85 and [SO 2]=0. 28?
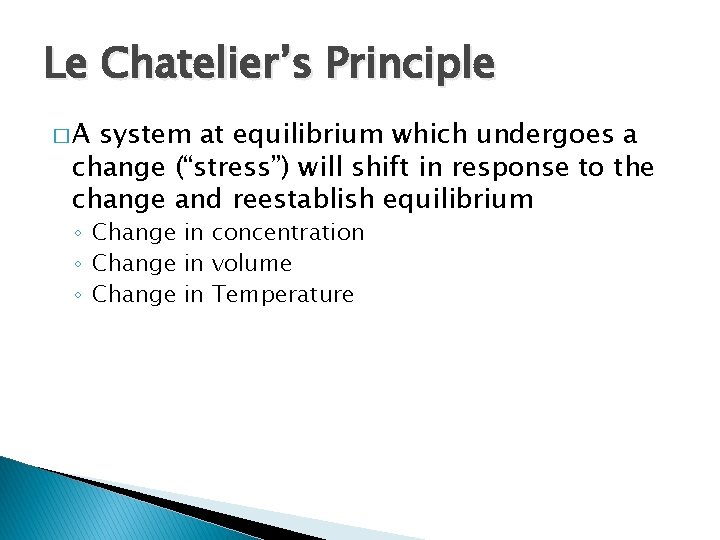
Le Chatelier’s Principle �A system at equilibrium which undergoes a change (“stress”) will shift in response to the change and reestablish equilibrium ◦ Change in concentration ◦ Change in volume ◦ Change in Temperature
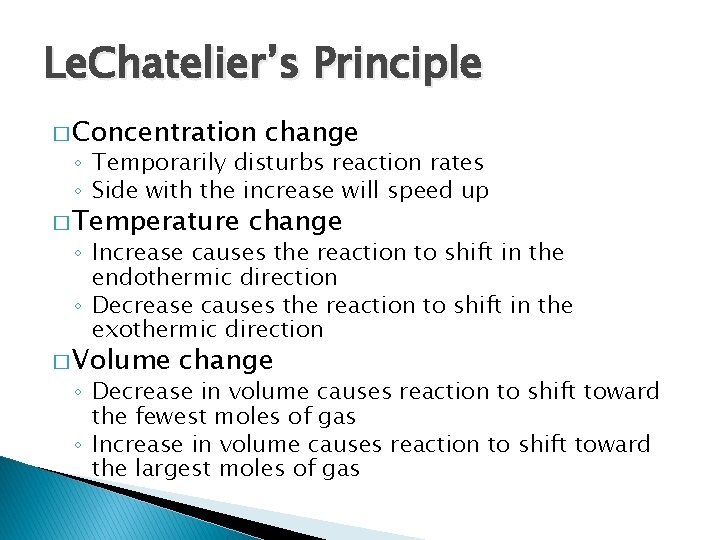
Le. Chatelier’s Principle � Concentration change ◦ Temporarily disturbs reaction rates ◦ Side with the increase will speed up � Temperature change ◦ Increase causes the reaction to shift in the endothermic direction ◦ Decrease causes the reaction to shift in the exothermic direction � Volume change ◦ Decrease in volume causes reaction to shift toward the fewest moles of gas ◦ Increase in volume causes reaction to shift toward the largest moles of gas
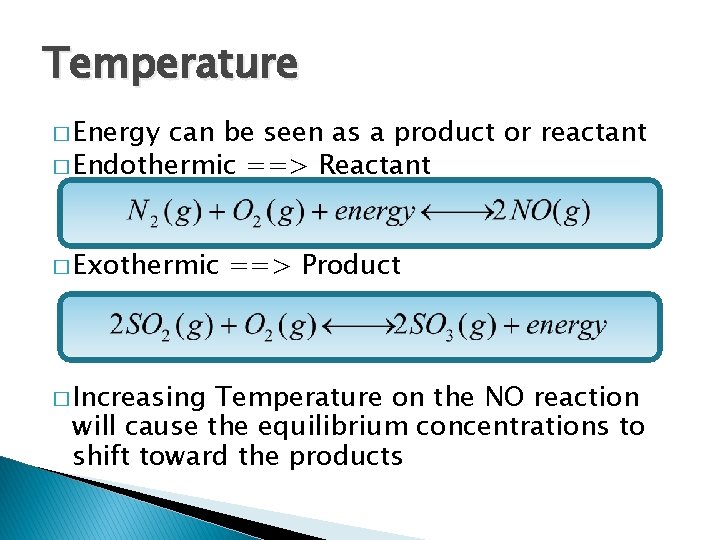
Temperature � Energy can be seen as a product or reactant � Endothermic ==> Reactant � Exothermic � Increasing ==> Product Temperature on the NO reaction will cause the equilibrium concentrations to shift toward the products
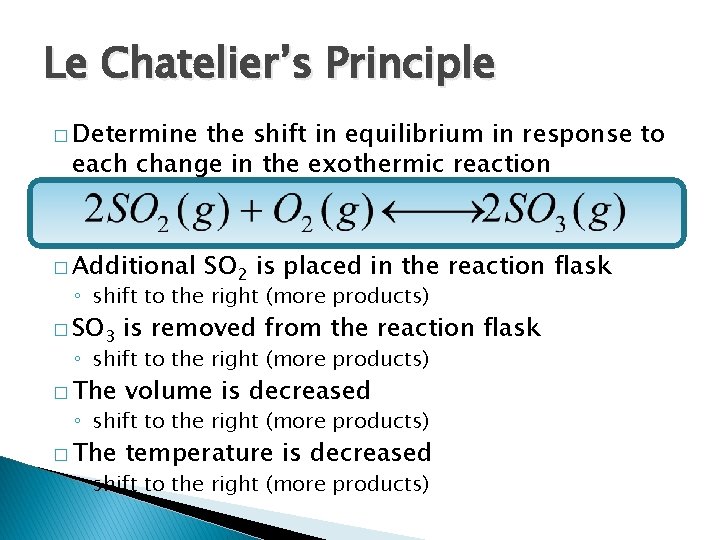
Le Chatelier’s Principle � Determine the shift in equilibrium in response to each change in the exothermic reaction � Additional SO 2 is placed in the reaction flask ◦ shift to the right (more products) � SO 3 is removed from the reaction flask � The volume is decreased � The temperature is decreased ◦ shift to the right (more products)

Solubility Product � Insoluble solubility solids actually have a very low molar ◦ Some actual solubility exists � Molar Solubility – the amount of dissolved solid present in a saturated solution
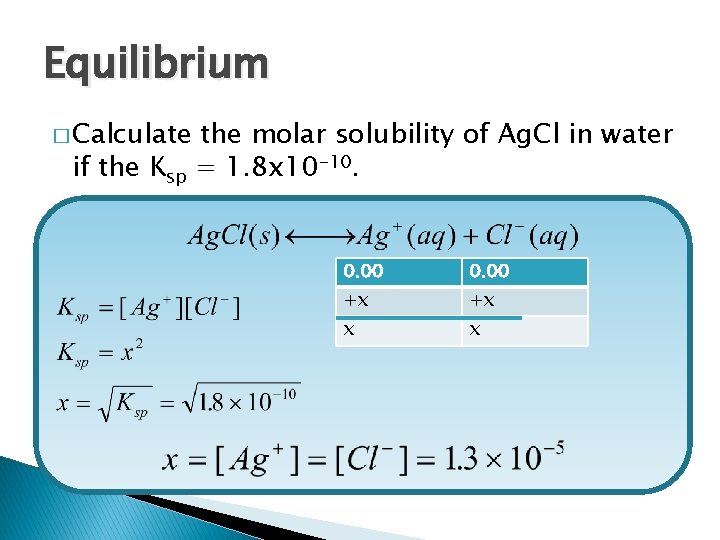
Equilibrium � Calculate if the Ksp the molar solubility of Ag. Cl in water = 1. 8 x 10 -10. 0. 00 +x +x x x
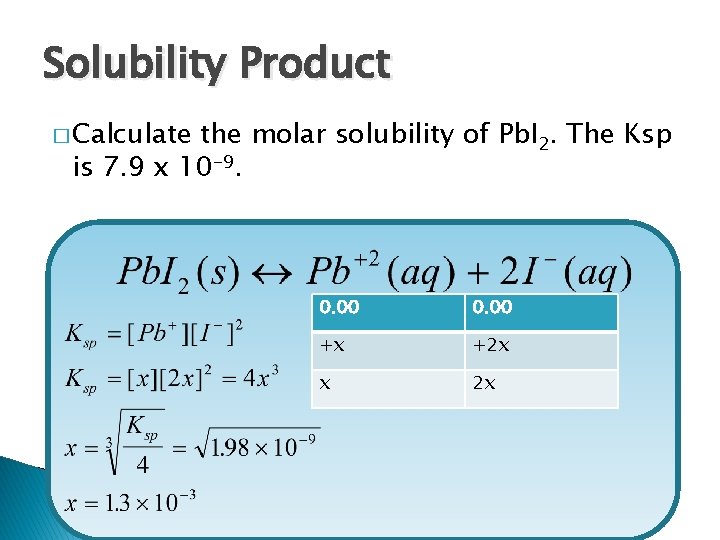
Solubility Product � Calculate the molar solubility of Pb. I 2. The Ksp is 7. 9 x 10 -9. 0. 00 +x +2 x x 2 x

Colligative Properties � Effect of a dissolved substance on properties of a solvent ◦ Vapor Pressure Lowering ◦ Boiling Point Elevation ◦ Freezing point Depression ◦ Osmotic Pressure
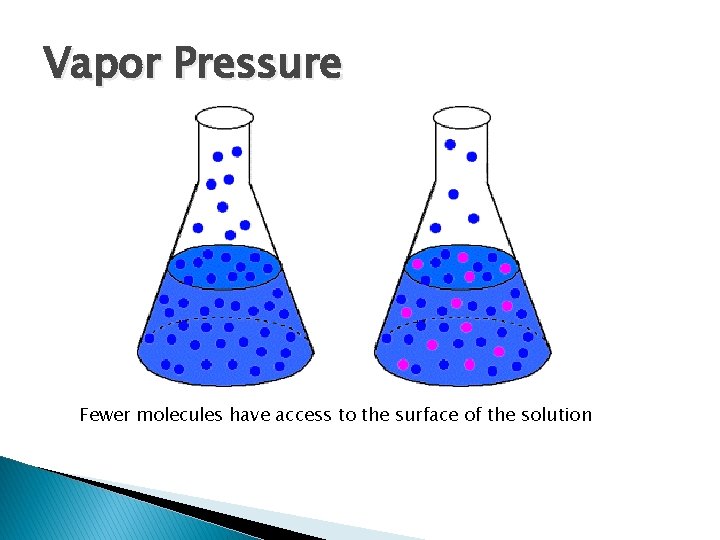
Vapor Pressure Fewer molecules have access to the surface of the solution
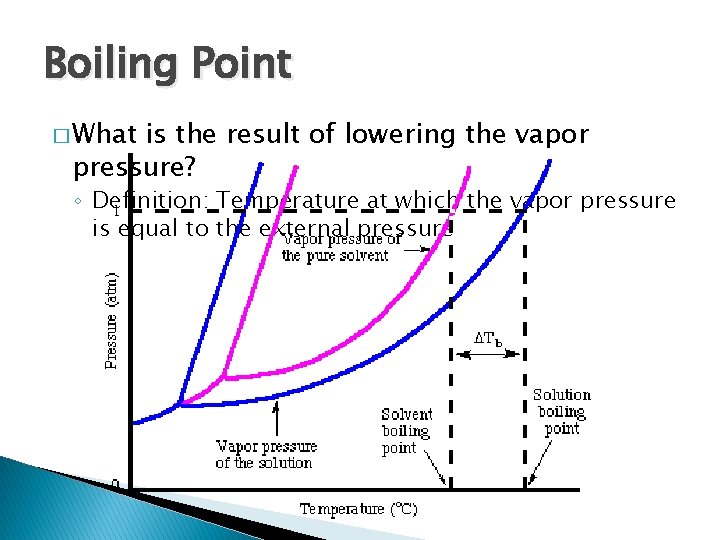
Boiling Point � What is the result of lowering the vapor pressure? ◦ Definition: Temperature at which the vapor pressure is equal to the external pressure

Freezing Point � Solute particles get in the way of making the solid ◦ Takes more energy to move them out of the way ◦ As water freezes, solution becomes more concentrated in salt/sugar ◦ Maximum temperature drop for a salt solution is -21. 1 °C (-6 °F).
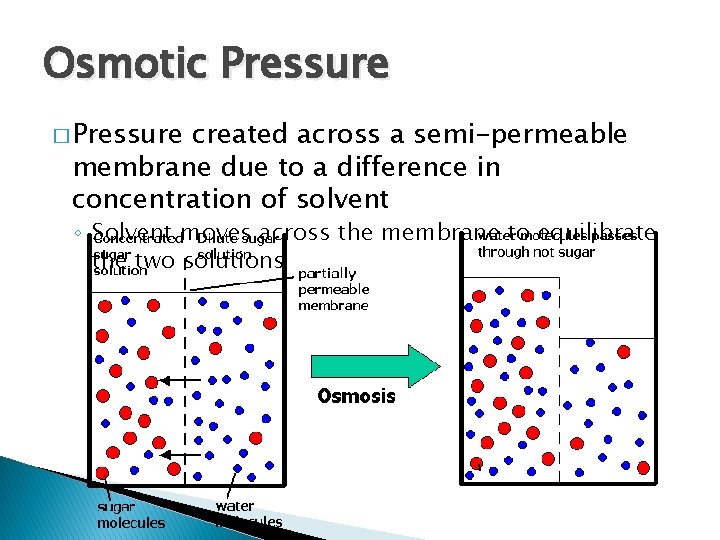
Osmotic Pressure � Pressure created across a semi-permeable membrane due to a difference in concentration of solvent ◦ Solvent moves across the membrane to equilibrate the two solutions
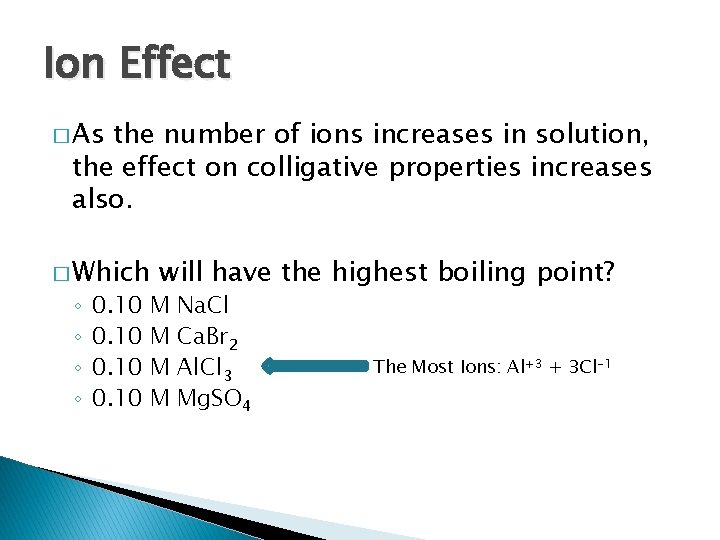
Ion Effect � As the number of ions increases in solution, the effect on colligative properties increases also. � Which ◦ ◦ 0. 10 will have the highest boiling point? M M Na. Cl Ca. Br 2 Al. Cl 3 Mg. SO 4 The Most Ions: Al+3 + 3 Cl-1
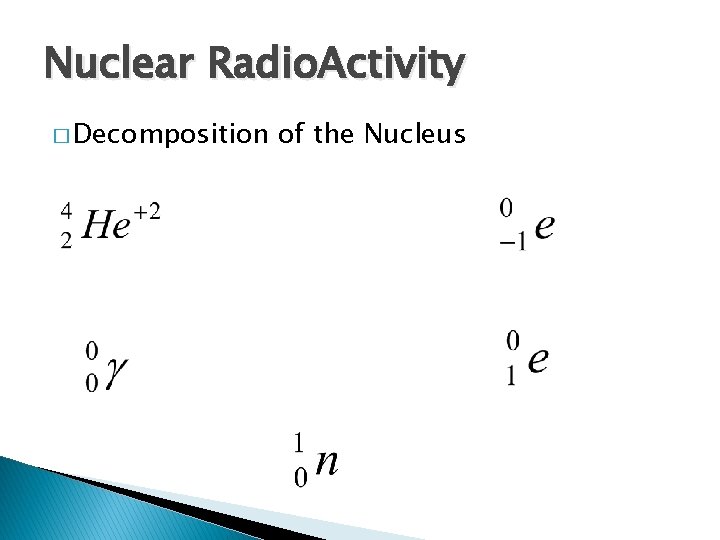
Nuclear Radio. Activity � Decomposition of the Nucleus
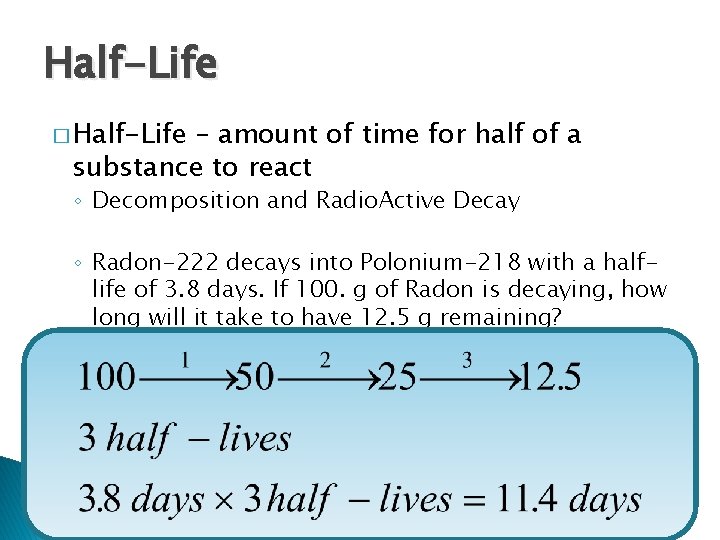
Half-Life � Half-Life – amount of time for half of a substance to react ◦ Decomposition and Radio. Active Decay ◦ Radon-222 decays into Polonium-218 with a halflife of 3. 8 days. If 100. g of Radon is decaying, how long will it take to have 12. 5 g remaining?
Chemistry � Organic Chemistry – Study of Carbon based molecules � Bio. Chemistry – Study of the Chemistry of Living Organisms � Why is Carbon the backbone atom of so many molecules? ◦ Relatively low EN – Covalent Bonds, even with metals
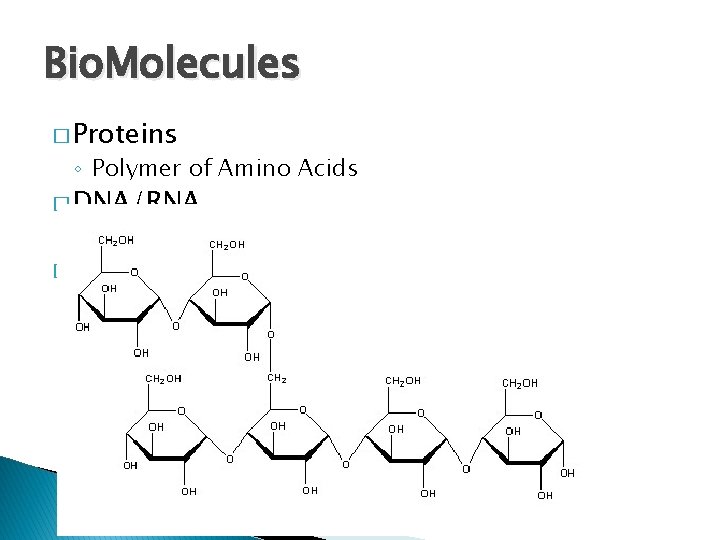
Bio. Molecules � Proteins ◦ Polymer of Amino Acids � DNA/RNA ◦ Polymer of Nucleic Acids � Starch/Glycogen ◦ Polymer of Glucose
- Slides: 32