Equilibrium Graphing Equilibrium vs The Collision Theory Lesson

Equilibrium Graphing Equilibrium vs. The Collision Theory

Lesson Outline Le Châtelier’s Principle according to The Collison-Reaction Theory Graphing Equilibrium Shifts

Decreasing Volume 2 SO 2(g) + 1 O 2(g) � 2 SO 3(g) ↓ volume results in an ↑ of both [products] and [reactants] Both forward and reverse reaction rates ↑ more moles of reactants results in more reactants colliding. Forward Reaction rate Increases As more products are produced, the forward reaction rate ↓ and the reverse reaction rate ↑ Equilibrium is reached
![Increasing [HCl] 2 HCl(aq) + 1 Zn(s) � 1 H 2(g) + 1 Zn. Increasing [HCl] 2 HCl(aq) + 1 Zn(s) � 1 H 2(g) + 1 Zn.](http://slidetodoc.com/presentation_image_h2/392effd522dfc221001e0137728e6b50/image-4.jpg)
Increasing [HCl] 2 HCl(aq) + 1 Zn(s) � 1 H 2(g) + 1 Zn. Cl 2(aq) ↑ [HCl] results in an increase of reactants colliding ↑ the forward reaction rate As Reactants React the forward reaction rate ↓ and As produces are produced the reverse reaction rate ↑ Equilibrium is reached
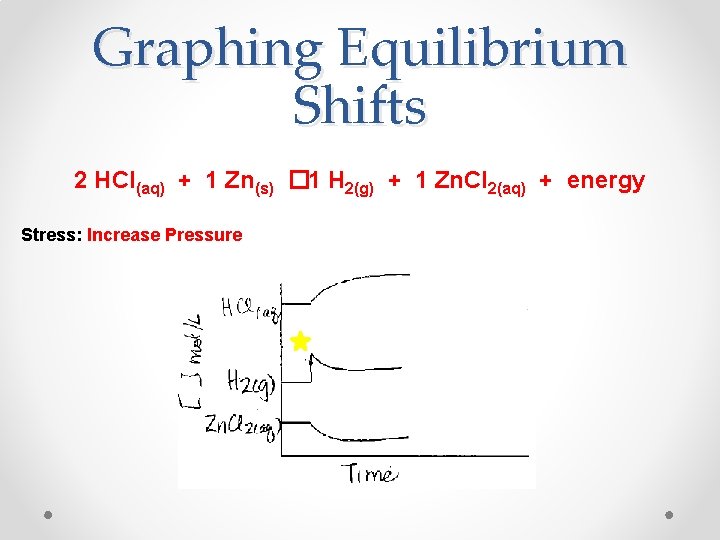
Graphing Equilibrium Shifts 2 HCl(aq) + 1 Zn(s) � 1 H 2(g) + 1 Zn. Cl 2(aq) + energy Stress: Increase Pressure
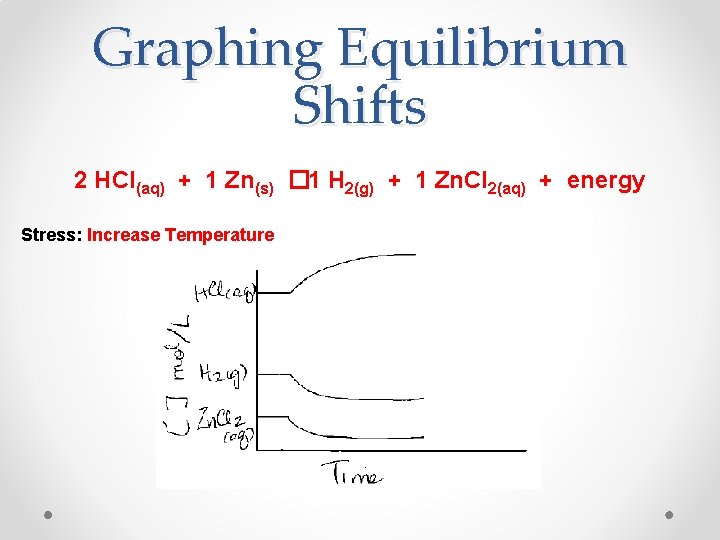
Graphing Equilibrium Shifts 2 HCl(aq) + 1 Zn(s) � 1 H 2(g) + 1 Zn. Cl 2(aq) + energy Stress: Increase Temperature
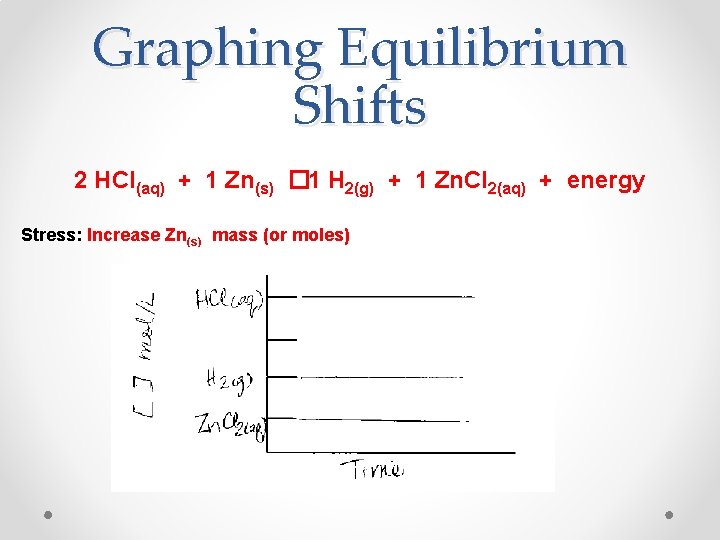
Graphing Equilibrium Shifts 2 HCl(aq) + 1 Zn(s) � 1 H 2(g) + 1 Zn. Cl 2(aq) + energy Stress: Increase Zn(s) mass (or moles)
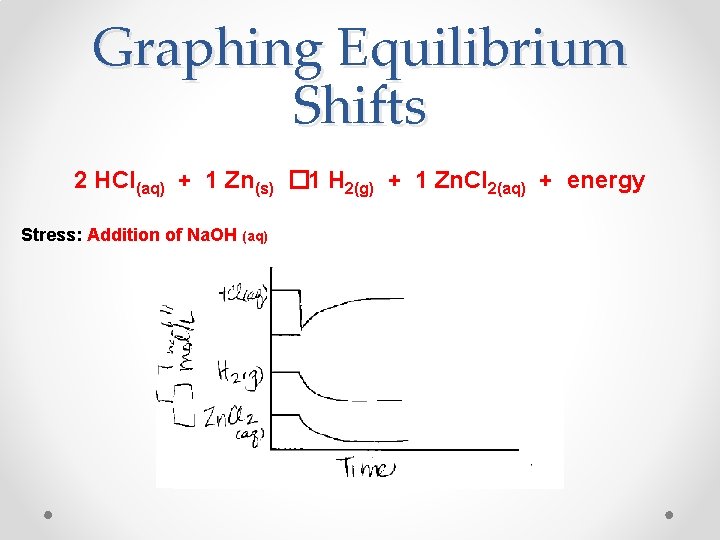
Graphing Equilibrium Shifts 2 HCl(aq) + 1 Zn(s) � 1 H 2(g) + 1 Zn. Cl 2(aq) + energy Stress: Addition of Na. OH (aq)
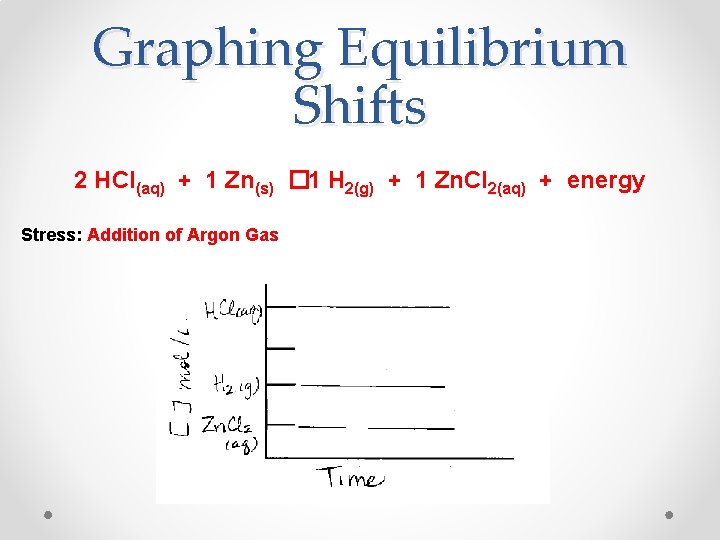
Graphing Equilibrium Shifts 2 HCl(aq) + 1 Zn(s) � 1 H 2(g) + 1 Zn. Cl 2(aq) + energy Stress: Addition of Argon Gas
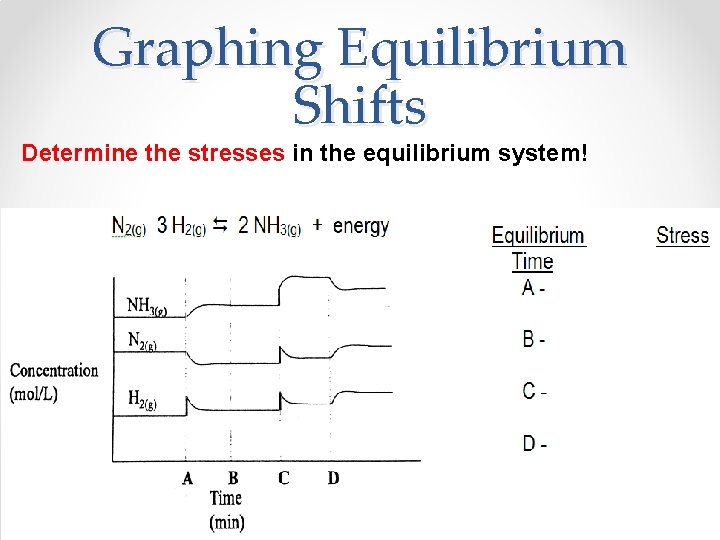
Graphing Equilibrium Shifts Determine the stresses in the equilibrium system!
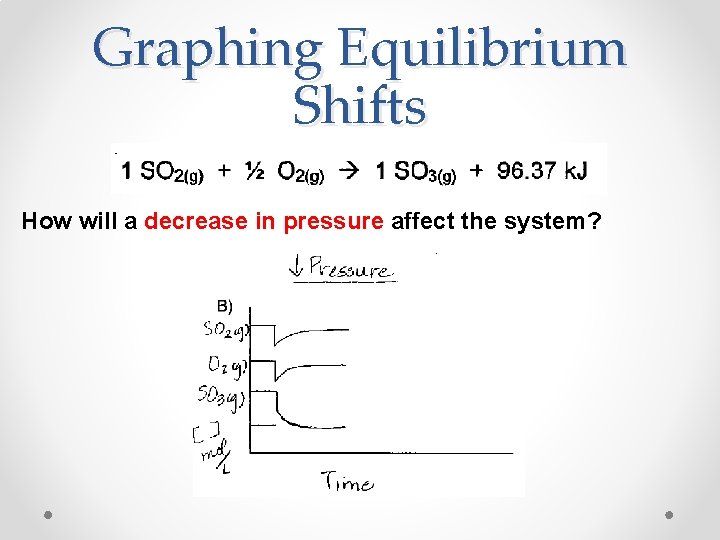
Graphing Equilibrium Shifts How will a decrease in pressure affect the system?

Looking Forward E 3: Graphing Equilibrium Shifts Le Châtelier’s Pre Laboratory (Friday) Le Châtelier’s Laboratory (Tuesday)
- Slides: 12