Equilibrium G AP Chemist ryThermod ynamics 3 Equilibrium

Equilibrium & ΔG AP Chemist ry-Thermod ynamics 3
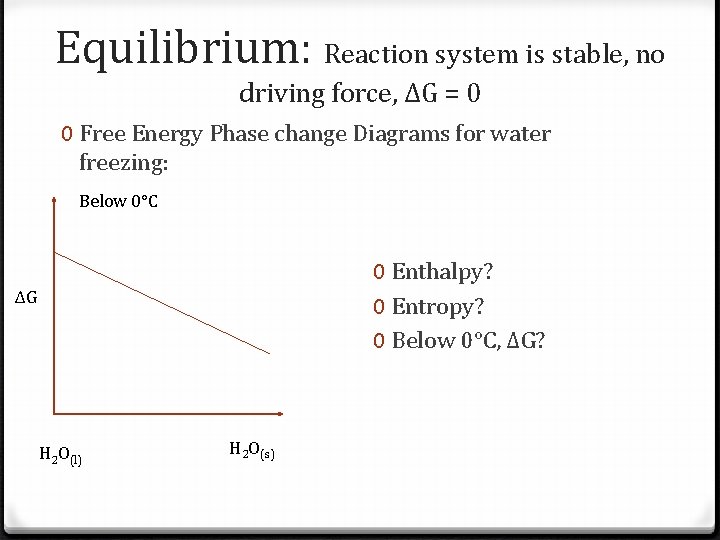
Equilibrium: Reaction system is stable, no driving force, ΔG = 0 0 Free Energy Phase change Diagrams for water freezing: Below 0°C 0 Enthalpy? 0 Entropy? 0 Below 0°C, ΔG? ΔG H 2 O(l) H 2 O(s)
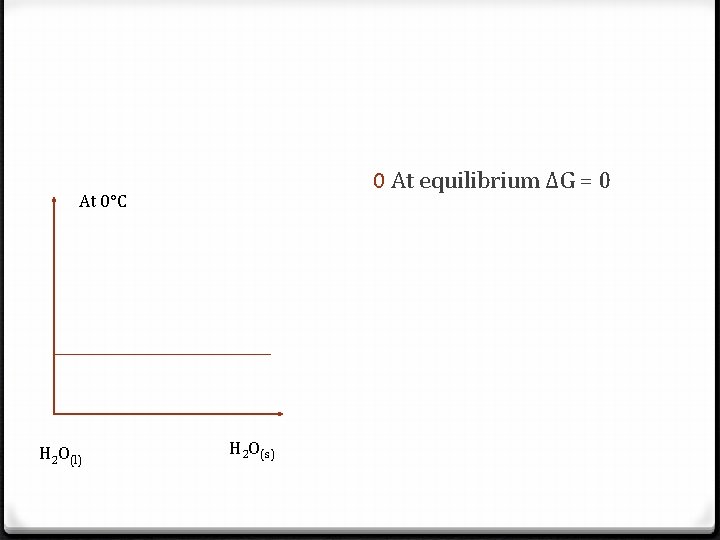
0 At equilibrium ΔG = 0 At 0°C H 2 O(l) H 2 O(s)
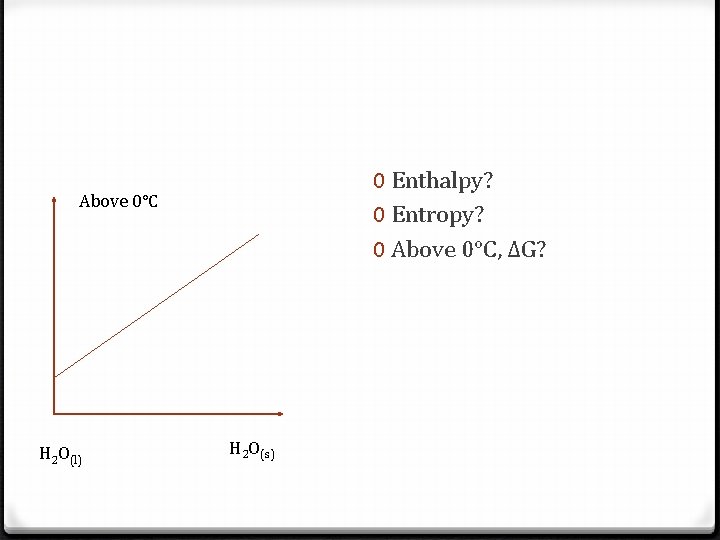
0 Enthalpy? 0 Entropy? 0 Above 0°C, ΔG? Above 0°C H 2 O(l) H 2 O(s)
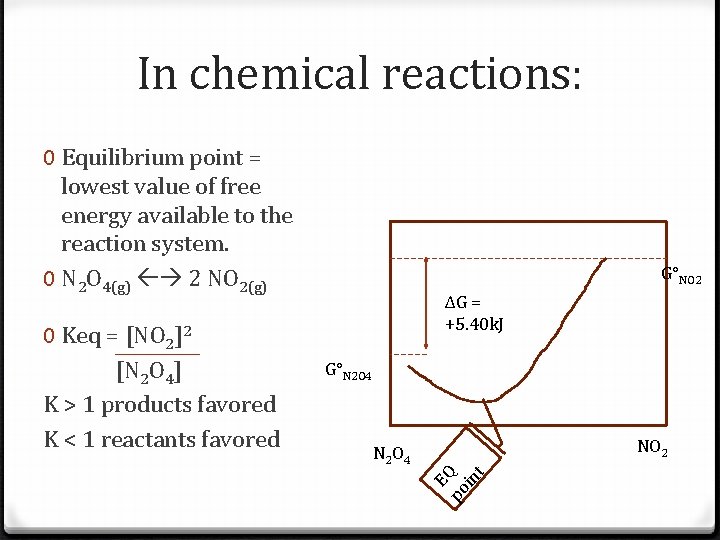
In chemical reactions: 0 Equilibrium point = lowest value of free energy available to the reaction system. 0 N 2 O 4(g) 2 NO 2(g) ΔG = +5. 40 k. J G°N 2 O 4 NO 2 po in t N 2 O 4 EQ 0 Keq = [NO 2]2 [N 2 O 4] K > 1 products favored K < 1 reactants favored G°NO 2
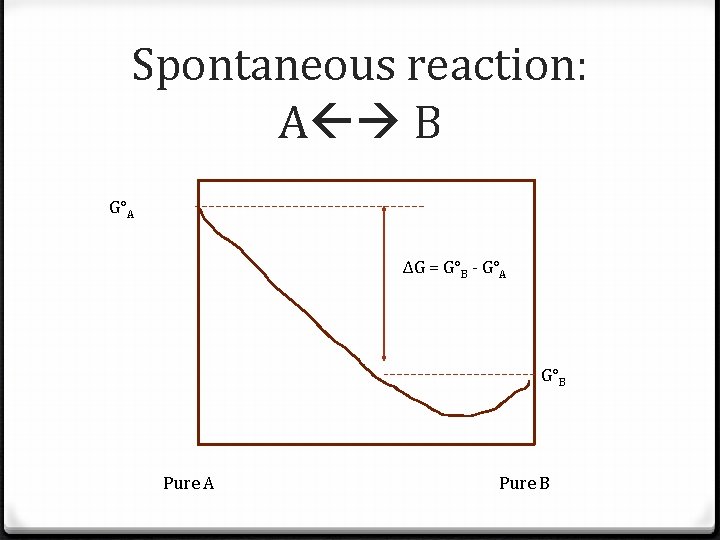
Spontaneous reaction: A B G°A ΔG = G°B - G°A G°B Pure A Pure B
![0 Solids & liquids are eliminated because their [EQ] don’t change significantly. 0 ΔG 0 Solids & liquids are eliminated because their [EQ] don’t change significantly. 0 ΔG](http://slidetodoc.com/presentation_image_h2/14a8d478d3d2b1b19c28a7bb0dd222fe/image-7.jpg)
0 Solids & liquids are eliminated because their [EQ] don’t change significantly. 0 ΔG = -RT ln Q 0 R = gas law constant = 8. 3145 J/K·mol 0 T = temp (K) 0 Ln = natural log 0 Q = initial concentrations of reagents

Keq can be calculated from ΔG°; at equilibrium ΔG = 0 0 ΔG = -RT ln K 0 K = thermodynamic equilibrium constant: “to what power we raise e to obtain the value of the equilibrium contant. ” 0 2 SO 2(g) + O 2(g) 2 SO 3(g) 0 ΔG = -33. 4 k. J at 25°C. Find K.
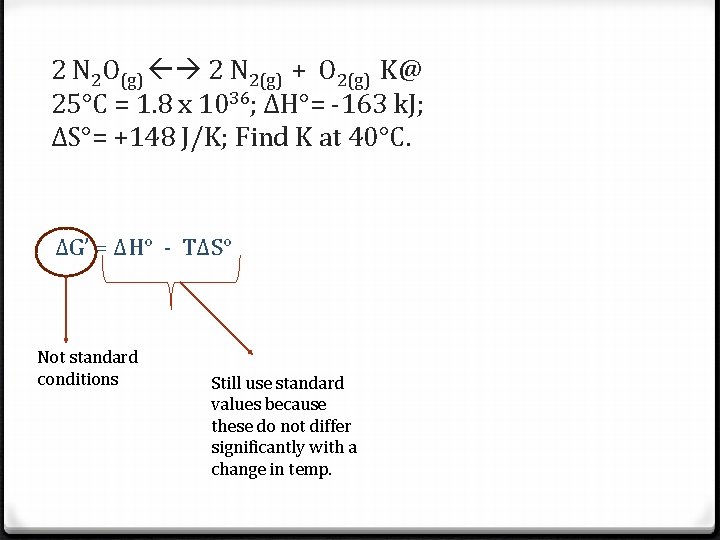
2 N 2 O(g) 2 N 2(g) + O 2(g) K@ 25°C = 1. 8 x 1036; ΔH°= -163 k. J; ΔS°= +148 J/K; Find K at 40°C. ΔG’ = ΔH° - TΔS° Not standard conditions Still use standard values because these do not differ significantly with a change in temp.
- Slides: 9