Equilibrium Expressions Nov 2017 The equilibrium reaction below

Equilibrium Expressions Nov 2017

The equilibrium reaction below was studied at 448 o. C: H 2(g) + I 2(g) 2 HI(g) Colourless Purple Colourless The following observations were made: • The purple colour never completely disappears • The same intensity of purple is present at the end of the reaction regardless of whether the reaction starts with, for example, 1 mole each of H 2 and I 2 or it starts with 2 moles of HI.
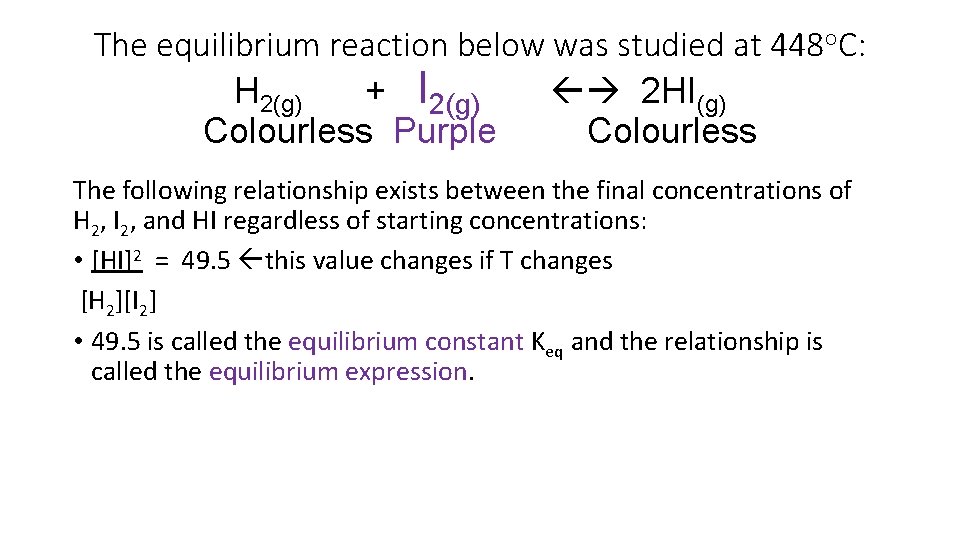
The equilibrium reaction below was studied at 448 o. C: H 2(g) + I 2(g) 2 HI(g) Colourless Purple Colourless The following relationship exists between the final concentrations of H 2, I 2, and HI regardless of starting concentrations: • [HI]2 = 49. 5 this value changes if T changes [H 2][I 2] • 49. 5 is called the equilibrium constant Keq and the relationship is called the equilibrium expression.
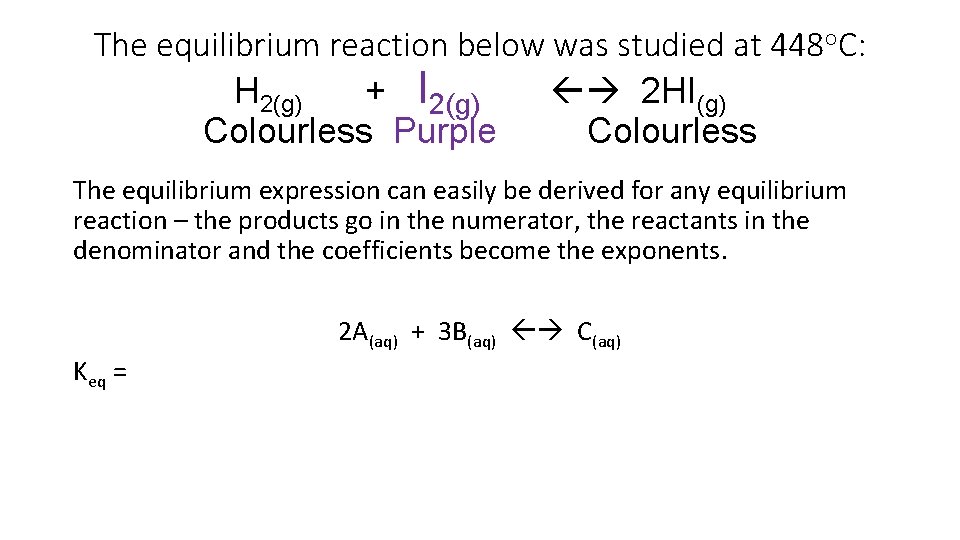
The equilibrium reaction below was studied at 448 o. C: H 2(g) + I 2(g) 2 HI(g) Colourless Purple Colourless The equilibrium expression can easily be derived for any equilibrium reaction – the products go in the numerator, the reactants in the denominator and the coefficients become the exponents. 2 A(aq) + 3 B(aq) C(aq) Keq =
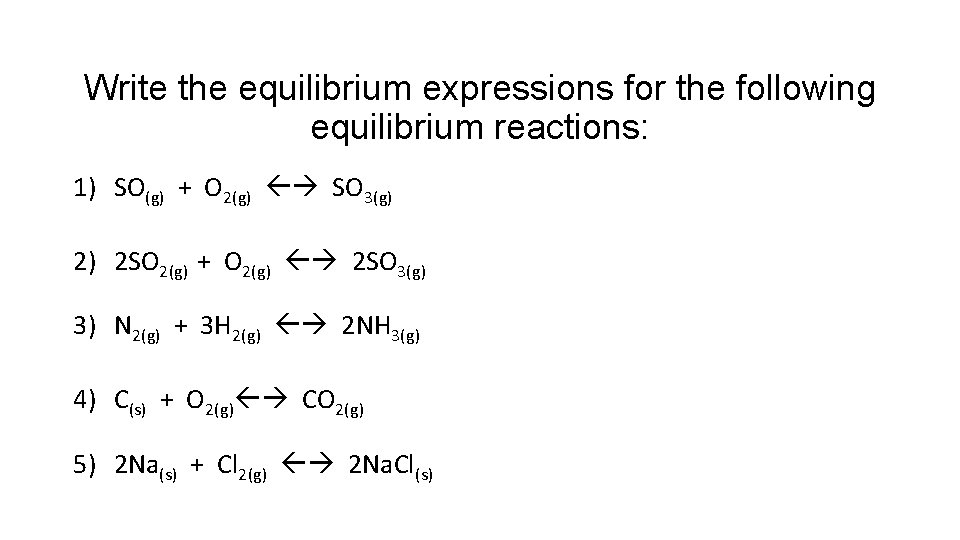
Write the equilibrium expressions for the following equilibrium reactions: 1) SO(g) + O 2(g) SO 3(g) 2) 2 SO 2(g) + O 2(g) 2 SO 3(g) 3) N 2(g) + 3 H 2(g) 2 NH 3(g) 4) C(s) + O 2(g) CO 2(g) 5) 2 Na(s) + Cl 2(g) 2 Na. Cl(s)
- Slides: 5