Equilibrium Equilibrium Constant Expressions Equilibrium Constant Expressions Solids

Equilibrium

Equilibrium Constant Expressions •

Equilibrium Constant Expressions • Solids are not written in expressions. • The concentration of a solid is unchanged and doesn’t affect the rate. • Water is not written in expressions. • The concentration is much higher for water, so it essentially stays the same.

Practice • Write equilibrium constant expressions for the following: • N 2(g) + 3 H 2(g) ⇌ 2 NH 3(g) • H 2 CO 3(aq) + H 2 O(l) ⇌ HCO 3 -(aq) + H 3 O+(aq)

Kc and Kp • There are various equilibrium constants, but the two we will look at now are about concentration and pressure. • Kc should be used if given the concentrations of substances. • If you get pressures, you will use Kp instead. They are not the same.

Meaning of K • What values would you expect for K if the reaction is product favored? • What if the reaction is reactant favored?

Assignment • Page 729 Exercise 16. 1 • Page 752 2

Reaction Quotient •

Practice • Nitrogen dioxide can exist in equilibrium with N 2 O 4. • 2 NO 2(g) ⇌ N 2 O 4(g) K=170 • If the concentration of NO 2 is 0. 015 M and the concentration of N 2 O 4 is 0. 025 M. Is Q larger than, smaller than, or equal to K? If not at equilibrium, what direction does the reaction need to proceed?
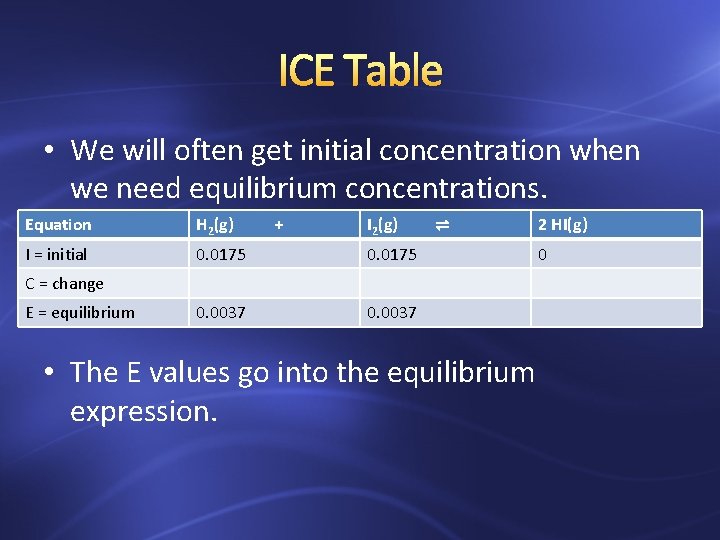
ICE Table • We will often get initial concentration when we need equilibrium concentrations. Equation H 2(g) + I 2(g) I = initial 0. 0175 0. 0037 ⇌ C = change E = equilibrium • The E values go into the equilibrium expression. 2 HI(g) 0

Example • An aqueous solution of ethanol and acetic acid, each at 0. 810 M initially, is heated to 100 °C. At equilibrium, the acetic acid concentration is 0. 748 M. Calculate K. • C 2 H 5 OH(aq) + CH 3 CO 2 H(aq) ⇌ CH 3 CO 2 C 2 H 5(aq) + H 2 O(l)

Assignment • Page 734 Exercise 16. 3 and 16. 4 • Page 736 Exercise 16. 5 • Page 752 6, 10, 12

Example: Equilibrium Concentration • Kc = 55. 64 for the equation H 2(g) + I 2(g) ⇌ 2 HI (g). If 1. 00 mol of each reactant are placed in a 0. 500 L flask. What are the concentrations for everything at equilibrium?

Practice • At a different temperature, Kc = 33 for the reaction H 2(g) + I 2(g) ⇌ 2 HI (g). If the initial concentrations are 6. 00 X 10 -3 M, find the equilibrium concentrations for all substances.

Quadratic Equilibrium • Some equations will give an expression that is quadratic. The quadratic formula can be used to solve. • If the K is small, more than 100 times smaller than the initial concentration, then the x on the bottom can be cancelled out. • If K is small, then the equilibrium is strongly reactant favored, so x will be infinitesimally small.

Example • N 2(g) + O 2(g) ⇌ 2 NO(g). At 1500 K, K = 1 X 10 -5. If a sample is 0. 80 M for nitrogen and 0. 20 M for oxygen, what are the equilibrium concentrations of reactants and products?

Assignment • Page 741 Exercise 16. 7 • Page 753 14, 15, 16

Equations and K • C(s) + ½ O 2(g) ⇌ CO(g) For this equation, K = 4. 6 X 1023 at 25 °C. What would K be if we instead balanced the equation as 2 C(s) + O 2(g) ⇌ 2 CO(g)?

Another Example • HCO 2 H(aq) + H 2 O(l) ⇌ HCO 2 -(aq) + H 3 O+(aq) • If the K for the forward reaction is 1. 8 X 10 -4, what is the K for the reverse reaction?

Practice • N 2(g) + 3 H 2(g) ⇌ 2 NH 3(g) K = 3. 5 X 108 • ½ N 2(g) + 3/2 H 2(g) ⇌ NH 3(g) K = • 2 NH 3(g) ⇌ N 2(g) + 3 H 2(g) K =

Combining Equations • When two equations are added, their Ks are multiplied to get the K for the combined equation. Ag. Cl(s) ⇌ Ag+(aq) + Cl-(aq) K 1 = 1. 8 X 10 -10 Ag+(aq) + 2 NH 3(aq) ⇌ [Ag(NH 3)2]+(aq) K 2 = 1. 6 X 107 Ag. Cl(s) + 2 NH 3(aq) ⇌ [Ag(NH 3)2]+(aq) + Cl-(aq) K = K 1 X K 2 = 1. 8 X 10 -10 X 1. 6 X 107 = 2. 9 X 10 -3

Assignment • Page 743 Exercises 16. 8 and 16. 9 • Page 754 20, 22, 24

Le Chatelier’s Principle • When a factor like temperature, concentration, or volume is changed, the equilibrium conditions will shift to counteract the change. • The concentrations of all reactants and products will change to make the reaction quotient equal to K again.
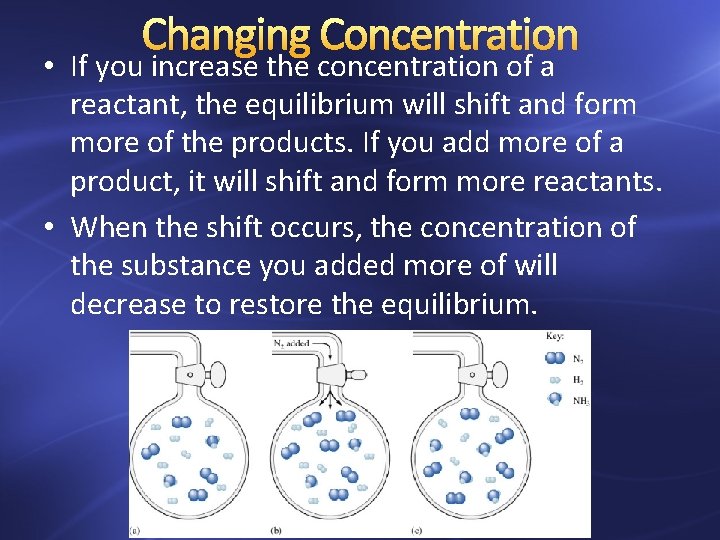
Changing Concentration • If you increase the concentration of a reactant, the equilibrium will shift and form more of the products. If you add more of a product, it will shift and form more reactants. • When the shift occurs, the concentration of the substance you added more of will decrease to restore the equilibrium.

Example: Butane ⇌ Isobutane, K=2. 50 • At equilibrium, the concentration of butane is 0. 500 M and the concentration of isobutene is 1. 25 M. If you add 1. 50 mol butane, what are the new equilibrium concentrations?

Changing Volume • Changing the volume may or may not affect the equilibrium. We can determine if it will or not based on the equation. • 2 NO 2(g) ⇌ N 2 O 4(g) • The above equation will shift to the right if we decrease the volume. It will favor the side with less gas molecules. • It will shift to the left if we increase the volume. It will favor the side with more gas molecules. • If the number of molecules are the same on each side, the equilibrium won’t change.
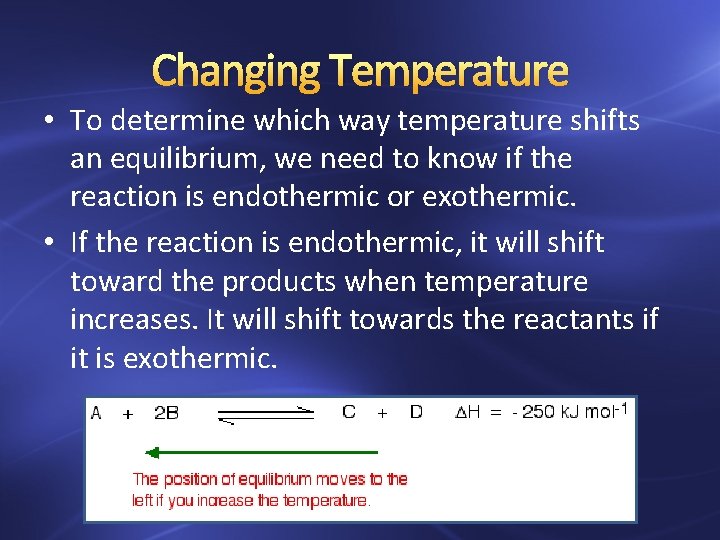
Changing Temperature • To determine which way temperature shifts an equilibrium, we need to know if the reaction is endothermic or exothermic. • If the reaction is endothermic, it will shift toward the products when temperature increases. It will shift towards the reactants if it is exothermic.

Temperature and K • Changing the temperature changes the K. • When temperature increases, K increases for an endothermic reaction since more products are formed. • When temperature increases, K decreases for an exothermic reaction since more reactants are formed.

Assignment • • Page 746 Exercise 16. 10 Page 747 Exercise 16. 11 Page 750 Exercise 16. 12 Page 754 26, 28, 30, 32, 36, 64, 68
- Slides: 29