Equilibrium Constant Keq Keq Is a value expressing

Equilibrium Constant (Keq)
![Keq: Is a value expressing the ratio of [products] & [reactants] at equilibrium constant Keq: Is a value expressing the ratio of [products] & [reactants] at equilibrium constant](http://slidetodoc.com/presentation_image_h/0933fdba5d3b792818e8e20aea8676d7/image-2.jpg)
Keq: Is a value expressing the ratio of [products] & [reactants] at equilibrium constant for every reversible rxn at equilibrium (@ constant T & P) a. A + b. B c. C + d. D Keq = [C]c[D]d [A]a[B]b ** pure liquids & solids are not included in the rate expression because their effective concentrations do not change
![Magnitude of Keq = [Products] [Reactants] Keq = 1 means products = reactants Keq Magnitude of Keq = [Products] [Reactants] Keq = 1 means products = reactants Keq](http://slidetodoc.com/presentation_image_h/0933fdba5d3b792818e8e20aea8676d7/image-3.jpg)
Magnitude of Keq = [Products] [Reactants] Keq = 1 means products = reactants Keq > 1 Forward rxn is favoured, [products] > [reactants] Keq < 1 reverse rxn is favoured, [products] < [reactants]
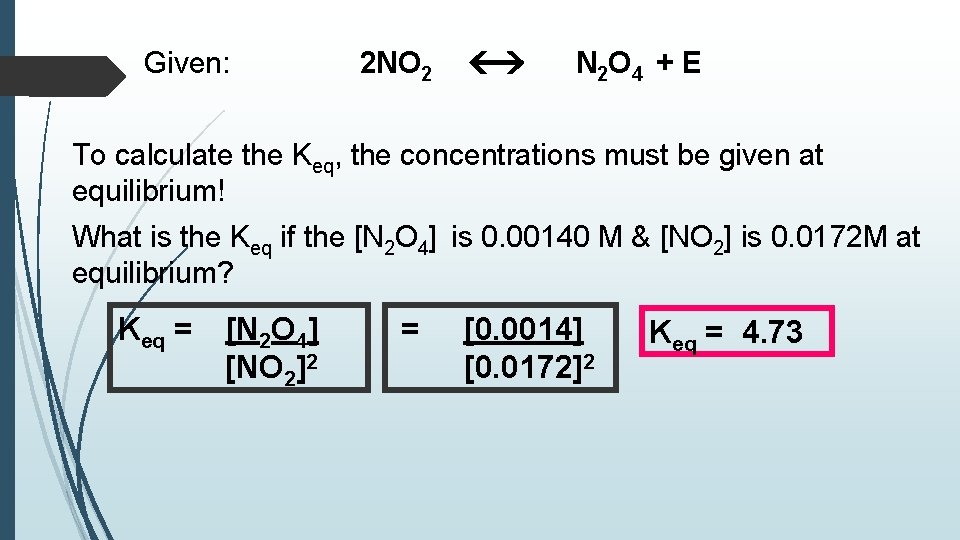
Given: 2 NO 2 N 2 O 4 + E To calculate the Keq, the concentrations must be given at equilibrium! What is the Keq if the [N 2 O 4] is 0. 00140 M & [NO 2] is 0. 0172 M at equilibrium? Keq = [N 2 O 4] [NO 2]2 = [0. 0014] [0. 0172]2 Keq = 4. 73
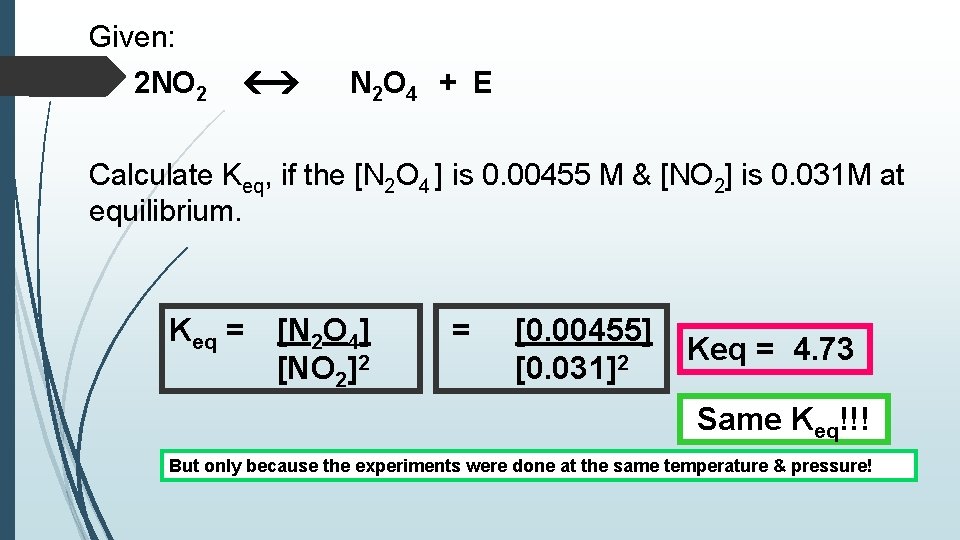
Given: 2 NO 2 N 2 O 4 + E Calculate Keq, if the [N 2 O 4 ] is 0. 00455 M & [NO 2] is 0. 031 M at equilibrium. Keq = [N 2 O 4] [NO 2]2 = [0. 00455] [0. 031]2 Keq = 4. 73 Same Keq!!! But only because the experiments were done at the same temperature & pressure!

Important! Keq is the same for a given reaction that is: at equilibrium at the same temperature & pressure no matter what the initial concentrations were.

Ex. 1 H 2 (g) + I 2 (g) ⇄2 HI (g) Find the Keq if the [H 2] is 0. 46 M, [I 2] is 0. 39 M & [HI] is 3. 0 M at equilibrium. Keq = [HI]2__ [H 2][I 2] = __[3. 0]2__ [0. 46][0. 39] Keq = 50

Ex. 2 PCl 5 (g) PCl 3 (g) + Cl 2 (g) Find the Keq, if the initial [PCl 5]is 0. 70 M & the equilibrium [Cl 2] is 0. 15 M. Keq expression for equilibrium concentrations only!!! Use an I-C-E table
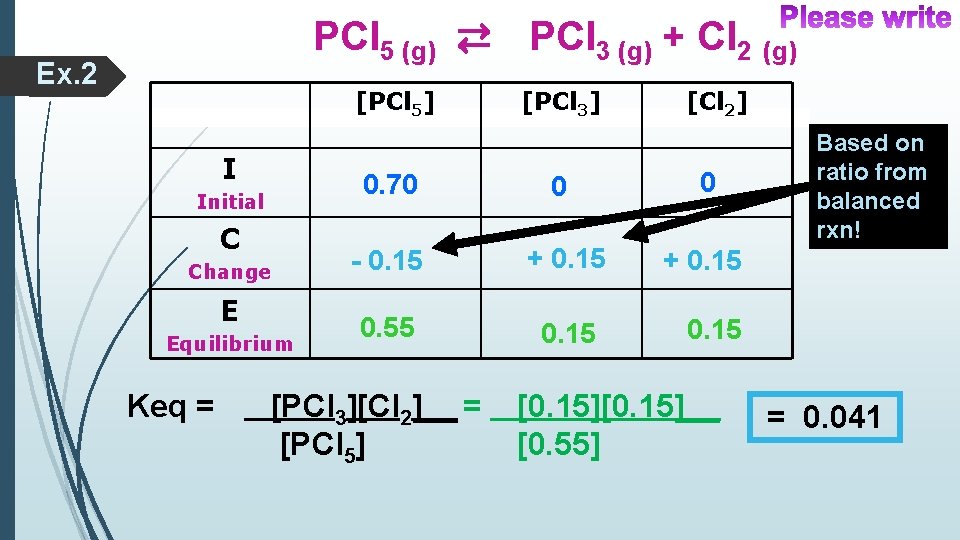
PCl 5 (g) ⇄ PCl 3 (g) + Cl 2 (g) Ex. 2 [PCl 5] I Initial C Change E Equilibrium Keq = [PCl 3] [Cl 2] 0. 70 0 0 - 0. 15 + 0. 15 0. 55 0. 15 [PCl 3][Cl 2]__ = [PCl 5] Based on ratio from balanced rxn! 0. 15 [0. 15]__ [0. 55] = 0. 041

P 318 #1 to #7

Ex. 3 2 NH 3 (g) 3 H 2 (g) + 1 N 2 (g) Initially a 5. 0 L flask contains 0. 2 M NH 3 & 0. 08 M N 2. At equilibrium [NH 3] is 0. 156 M. Find Keq I C E NH 3 H 2 0 N 2 0. 08 - 0. 044 MOLE +RATIO 0. 066 + 0. 022 0. 156 0. 066 0. 102
![Keq = = [H 2]3[N 2]__ [NH 3]2 [0. 066]3[0. 102]__ [0. 156]2 Keq Keq = = [H 2]3[N 2]__ [NH 3]2 [0. 066]3[0. 102]__ [0. 156]2 Keq](http://slidetodoc.com/presentation_image_h/0933fdba5d3b792818e8e20aea8676d7/image-12.jpg)
Keq = = [H 2]3[N 2]__ [NH 3]2 [0. 066]3[0. 102]__ [0. 156]2 Keq = 0. 0012

Ex. 4 H 2 (g) + I 2 (g) 2 HI (g) Find the [HI] at equilibrium, if [H 2] is 0. 50 M & [I 2] is 0. 50 M at equilibrium (Keq = 50). Keq = [HI]2 [H 2] [I 2] 50 = [x]2__ [0. 50] [HI] = 3. 5 M at equilibrium
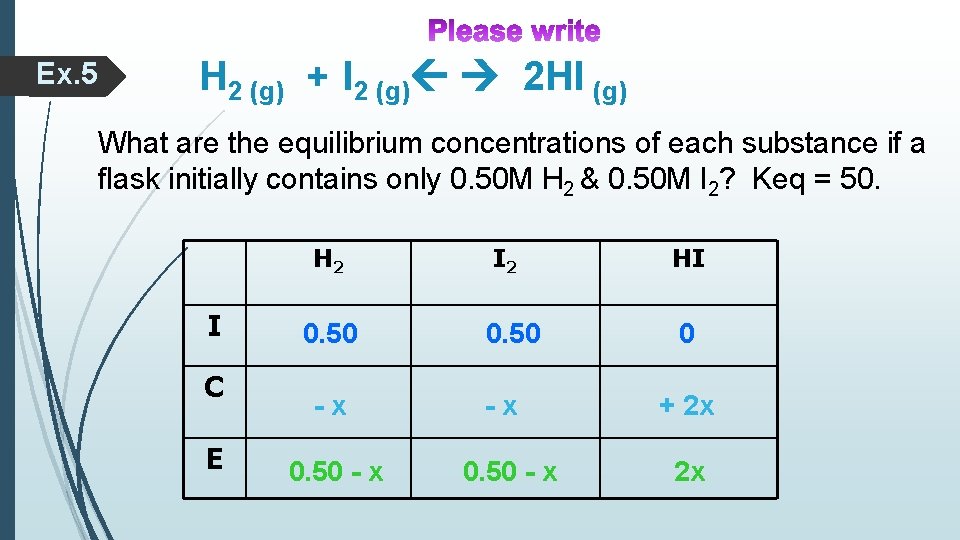
Ex. 5 H 2 (g) + I 2 (g) 2 HI (g) What are the equilibrium concentrations of each substance if a flask initially contains only 0. 50 M H 2 & 0. 50 M I 2? Keq = 50. H 2 I C E 0. 50 -x 0. 50 - x I 2 0. 50 -x 0. 50 - x HI 0 + 2 x 2 x
![At Equilibrium: [H 2] = 0. 50 -x = 0. 50 – 0. At Equilibrium: [H 2] = 0. 50 -x = 0. 50 – 0.](http://slidetodoc.com/presentation_image_h/0933fdba5d3b792818e8e20aea8676d7/image-15.jpg)
At Equilibrium: [H 2] = 0. 50 -x = 0. 50 – 0. 70 = - 0. 20 M!!! [H 2] = 0. 50 -x = 0. 50 – 0. 39 = 0. 11 M!!! [I 2] = [H 2] = 0. 11 M [HI] = 2 x = 2 x 0. 39 = 0. 78 M /
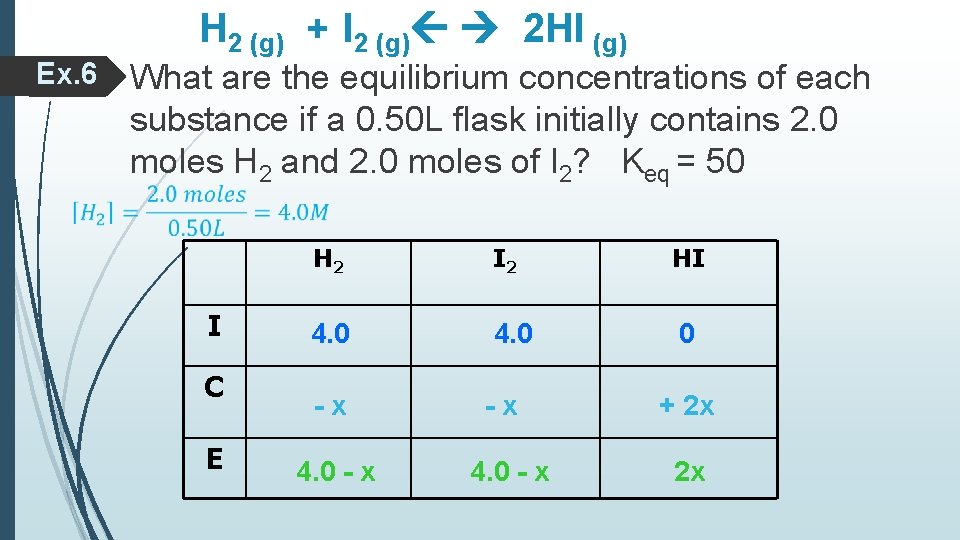
Ex. 6 H 2 (g) + I 2 (g) 2 HI (g) What are the equilibrium concentrations of each substance if a 0. 50 L flask initially contains 2. 0 moles H 2 and 2. 0 moles of I 2? Keq = 50 I C E H 2 I 2 4. 0 -x 4. 0 - x HI 0 + 2 x 2 x
![At Equilibrium: [H 2] = 4. 0 -x = 4. 0 - 3. At Equilibrium: [H 2] = 4. 0 -x = 4. 0 - 3.](http://slidetodoc.com/presentation_image_h/0933fdba5d3b792818e8e20aea8676d7/image-17.jpg)
At Equilibrium: [H 2] = 4. 0 -x = 4. 0 - 3. 12 = 0. 88 M [I 2] = 4. 0 -x = 4. 0 - 3. 12 = 0. 88 M [HI] = 2 x = 2 x 3. 12 = 6. 24 M If the exponents differ solve using the quadratic formula.

Ex. 7 At 1100 K the Keq = 25. H 2(g) + I 2(g) 2 HI(g) 2 moles of H 2 and 3 moles of I 2 are placed in a 1 L container. Find the concentration of each substance at equilibrium?
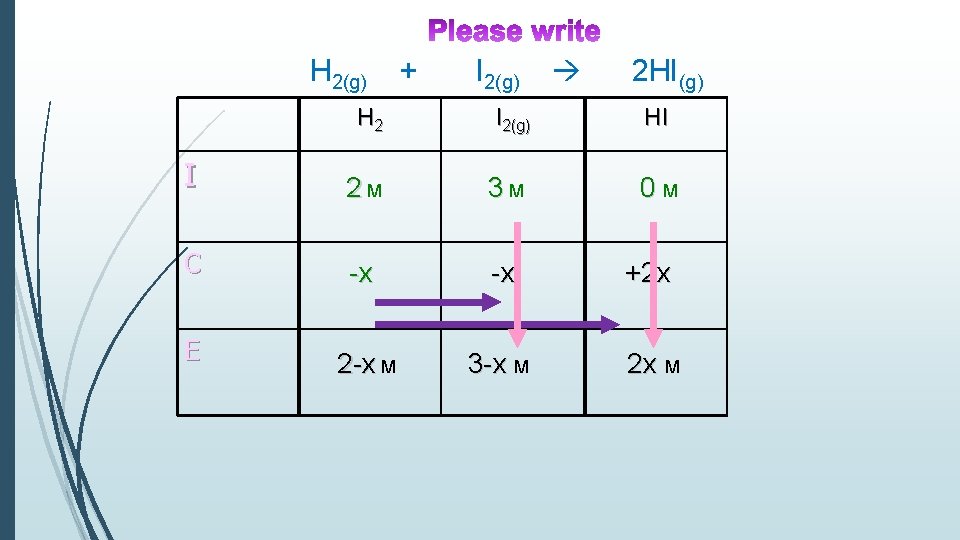
H 2(g) + I 2(g) 2 HI(g) H 2 I 2(g) HI I 2 M 3 M 0 M C -x +2 x E 2 -x M 3 -x M 2 x 2 x M
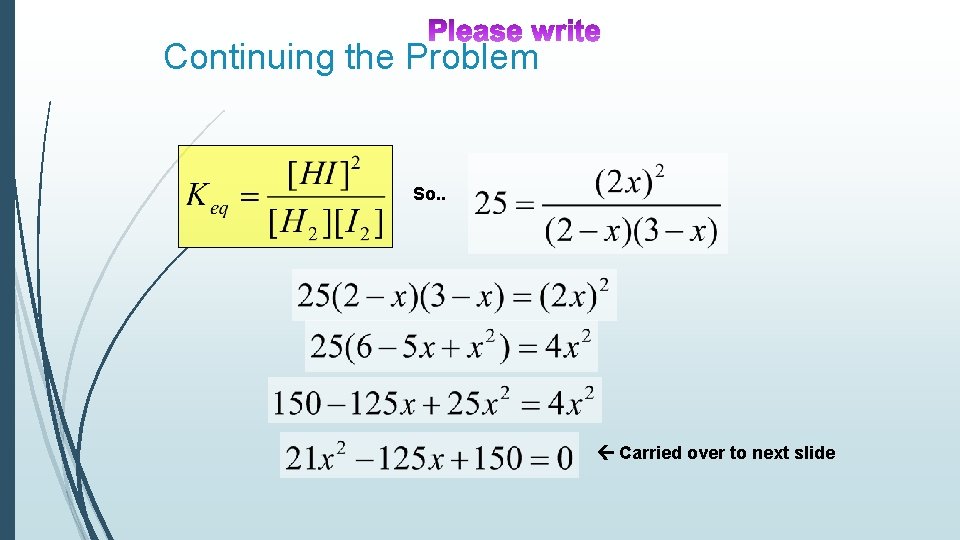
Continuing the Problem So. . Carried over to next slide

Using the Quadratic formula, where: a=21, b=-125, c=150 Two solutions to formula But only one of the solutions will give a real answer!

Choosing the Best Answers Two solutions to formula Try finding the correct concentrations using the solutions Solve using 4. 29 Solve using 1. 67 Concentration cannot be negative! At equilibrium: [H 2] = 0. 33 mol/L, [I 2] = 1. 33 mol/L and [HI] = 3. 34 mol/L
- Slides: 22