Equilibrium Collision model Molecules react by colliding with

Equilibrium

Collision model § Molecules react by colliding with each other. § If a compound has a higher concentration, then it has more molecules, and produces more collisions.

Activation Energy needed for a reaction to occur. If Ecollision>Eactivation, then a reaction will occur. If Ecollision<Eactivation, then a reaction will not occur. At higher temp, speed of molecules increases, so collisions have more energy, so the reaction is more likely.

Catalyst § You can speed up a reaction without increasing the temperature, this is called a catalyst. § A catalyst provides a new pathway for the reaction. Ex: traffic jam

Ozone § Our ozone layer is depleted because Cl acts as a catalyst to decompose the ozone. Cl + O 3 Cl. O + O 2 O + Cl. O Cl + O 2 O+O 3 2 O 2 § Notice that the overall amount of Cl doesn’t change.
The Basics § In equilibrium opposing processes occur at the same time and rate § The forward and reverse reactions continue to occur at equal rates in equilibrium.
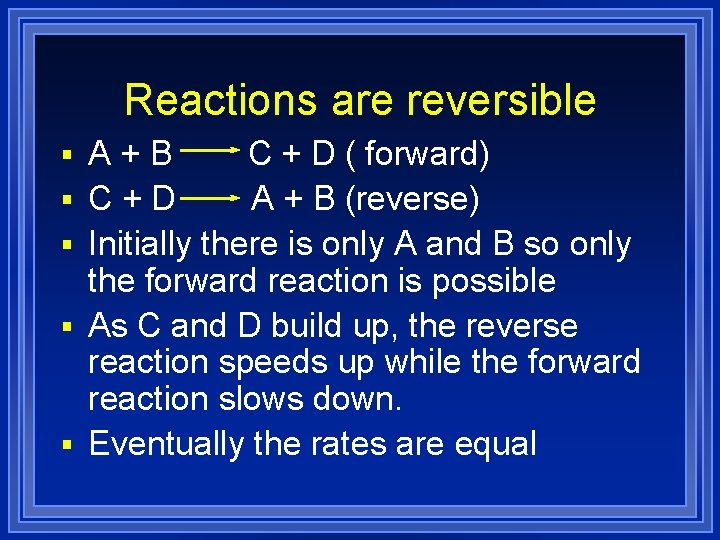
Reactions are reversible § A+B § § C + D ( forward) C+D A + B (reverse) Initially there is only A and B so only the forward reaction is possible As C and D build up, the reverse reaction speeds up while the forward reaction slows down. Eventually the rates are equal

§ Reversible reaction-a chemical reaction in which the products can react to re-form the reactants § ex: ice water non example: egg cooked egg
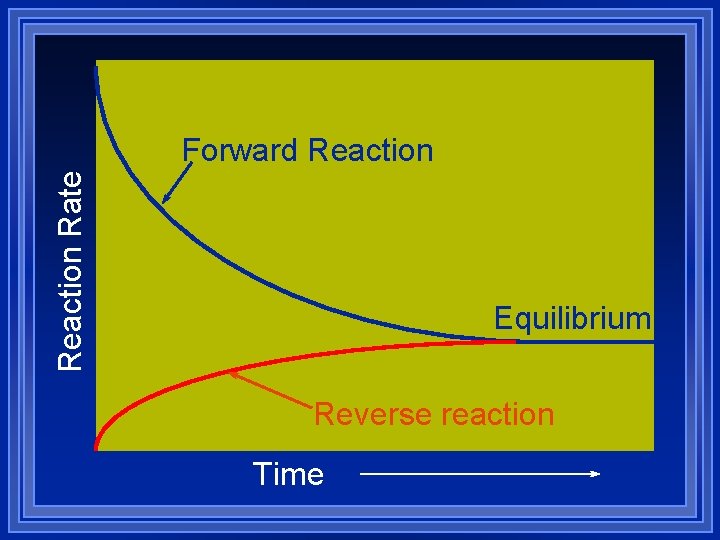
Reaction Rate Forward Reaction Equilibrium Reverse reaction Time

What is equal at Equilibrium? § Rates are equal. § Concentrations are not. § Rates are determined by concentrations and activation energy. § The concentrations do not change at equilibrium. § Ex: Traffic flow on a bridge
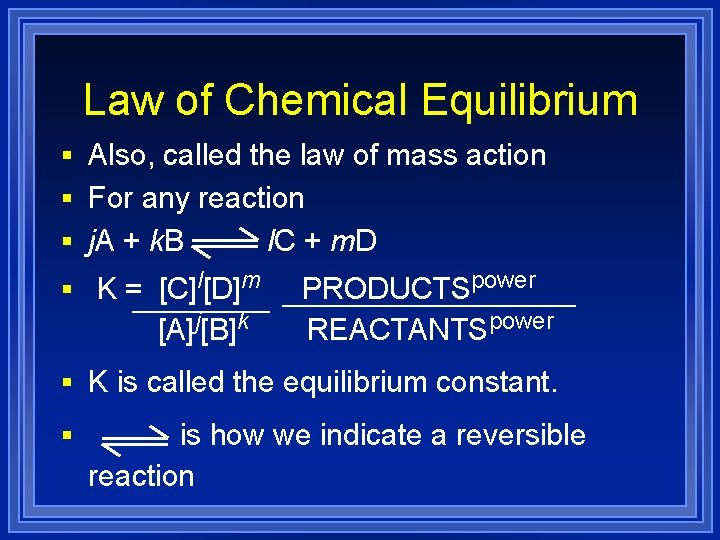
Law of Chemical Equilibrium § Also, called the law of mass action § For any reaction § j. A + k. B § K = [C]l[D]m [A]j[B]k l. C + m. D PRODUCTSpower REACTANTSpower § K is called the equilibrium constant. § is how we indicate a reversible reaction

K § § § If K=1, the products and reactants are equal concentrations If K is low, the reactants are favored If K is high, the products are favored (the larger K the more products formed)

Practice § Write the equilibrium constant expression for: § 2 SO 2(g) + O 2(g) 2 SO 3(g)
![Answer K [SO 3]2 = [SO 2]2 [O 2] Answer K [SO 3]2 = [SO 2]2 [O 2]](http://slidetodoc.com/presentation_image_h2/17df785e6864f2535c85225a17a90289/image-14.jpg)
Answer K [SO 3]2 = [SO 2]2 [O 2]

K is CONSTANT § At any temperature. § Temperature affects rate. § The equilibrium concentrations don’t have to be the same only K. § Equilibrium position is a set of concentrations at equilibrium. § There an unlimited number.

Heterogeneous Equilibria § So far every example dealt with reactants and products where all were in the same phase (homogeneous). § If the reaction involves pure solids or pure liquids the concentration of the solid or the liquid doesn’t change. § As long as they are not used up we can leave them out of the equilibrium expression.
![For Example § H 2(g) + I 2(s) § K = [HI]2 2 HI(g) For Example § H 2(g) + I 2(s) § K = [HI]2 2 HI(g)](http://slidetodoc.com/presentation_image_h2/17df785e6864f2535c85225a17a90289/image-17.jpg)
For Example § H 2(g) + I 2(s) § K = [HI]2 2 HI(g) [H 2][I 2] § But the concentration of I 2 does not change. § K= [HI]2 [ H 2]

Does this look familiar? § Ionization Constant of Water § H 2 O(l)+H 2 O(l) H 3 O+(aq)+OH- (aq) § Kw=[H 3 O+] [OH-] =1. 0 x 10 -14
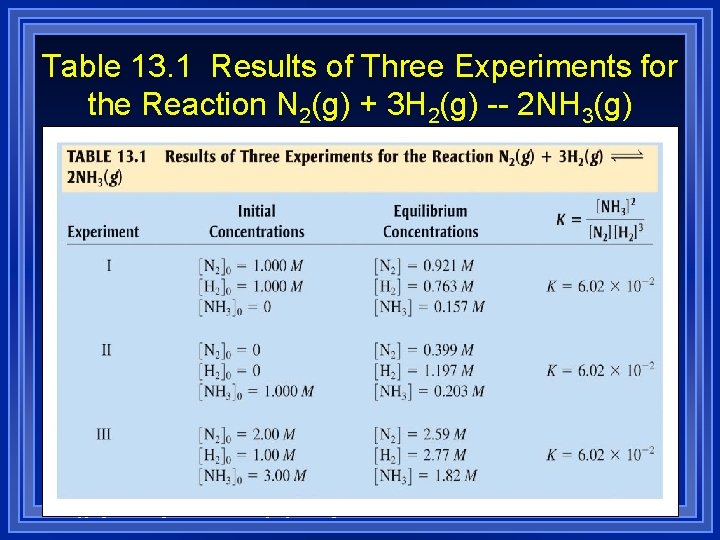
Table 13. 1 Results of Three Experiments for the Reaction N 2(g) + 3 H 2(g) -- 2 NH 3(g) Copyright © Houghton Mifflin Company. All rights reserved.

Le Chatelier’s Principle § If a stress is applied to a system at equilibrium, the position of the equilibrium will shift to reduce the stress. § Analogy: boat § 3 Types of stress

1) Change amounts of reactants and/or products § Adding product makes § Removing reactant makes § Adding reactant makes § Removing product makes

2) Change Pressure § By changing volume § System will move in the direction that has the least moles of gas. § Because partial pressures (and concentrations) change a new equilibrium must be reached. § System tries to minimize the moles of gas.

3) Change in Temperature § Affects the rates of both the forward and reverse reactions. § Doesn’t just change the equilibrium position, changes the equilibrium constant. § The direction of the shift depends on whether it is exo- or endothermic

Exothermic § DH<0 § Releases heat § Think of heat as a product § Raising temperature push toward reactants. § Shifts to left.

Endothermic § DH>0 § Absorbs heat § Think of heat as a reactant § Raising temperature push toward products. § Shifts to right.
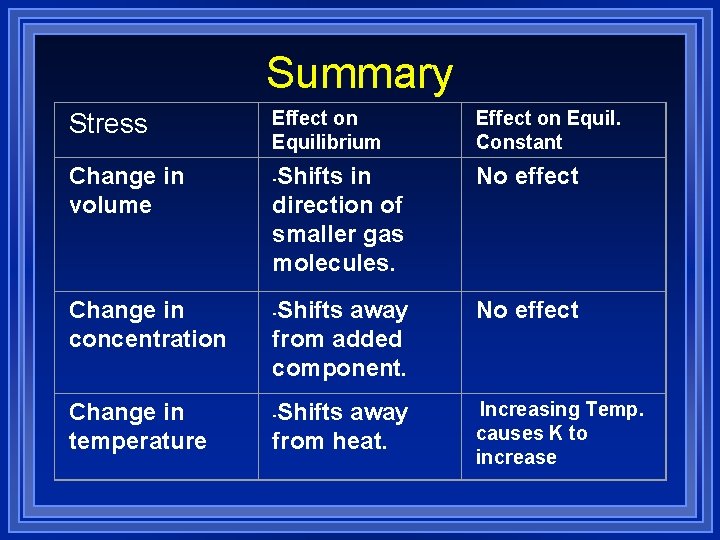
Summary Stress Change in volume Change in concentration Change in temperature Effect on Equilibrium Effect on Equil. Constant Shifts in direction of smaller gas molecules. No effect Shifts away from added component. No effect Shifts away from heat. Increasing Temp. causes K to increase - - -
- Slides: 26