Equilibrium Chapter 19 2 Reversible Reactions A reaction

Equilibrium Chapter 19 -2

Reversible Reactions • A reaction in which the conversion of reactants into products and the conversion of products into reactants occur simultaneously
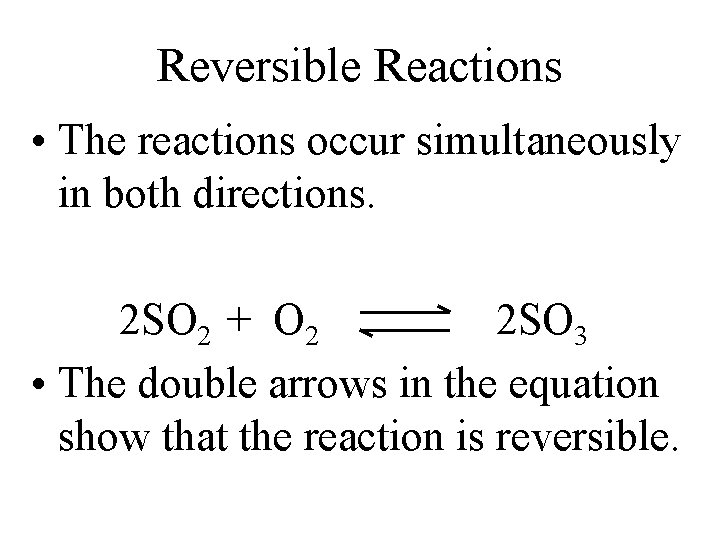
Reversible Reactions • The reactions occur simultaneously in both directions. 2 SO 2 + O 2 2 SO 3 • The double arrows in the equation show that the reaction is reversible.

Chemical equilibrium • It is a state in which the forward reaction and reverse reaction takes place at the same rate.

Le Châtelier’s Principle • When stress is applied to a system at equilibrium, the system changes to relieve the stress. • It is used to predict the changes that occur when a system at equilibrium is disturbed.
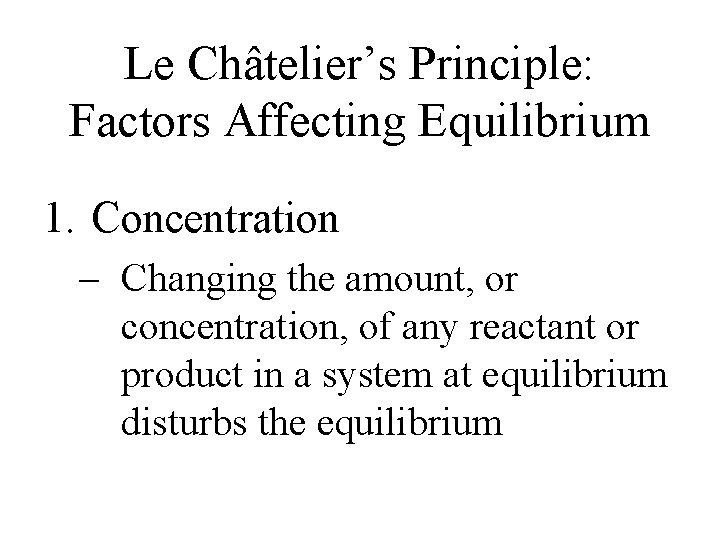
Le Châtelier’s Principle: Factors Affecting Equilibrium 1. Concentration – Changing the amount, or concentration, of any reactant or product in a system at equilibrium disturbs the equilibrium
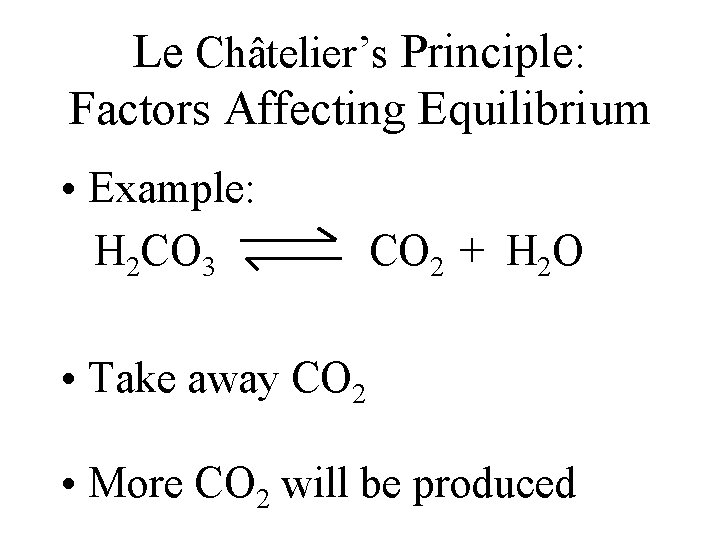
Le Châtelier’s Principle: Factors Affecting Equilibrium • Example: H 2 CO 3 CO 2 + H 2 O • Take away CO 2 • More CO 2 will be produced
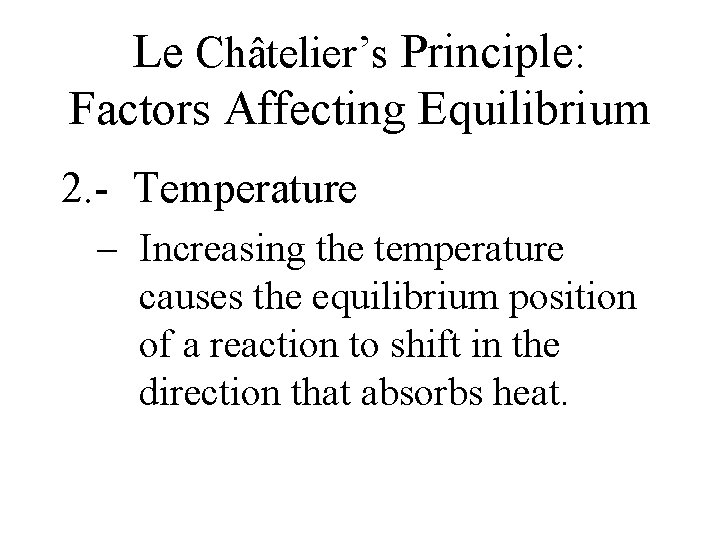
Le Châtelier’s Principle: Factors Affecting Equilibrium 2. - Temperature – Increasing the temperature causes the equilibrium position of a reaction to shift in the direction that absorbs heat.
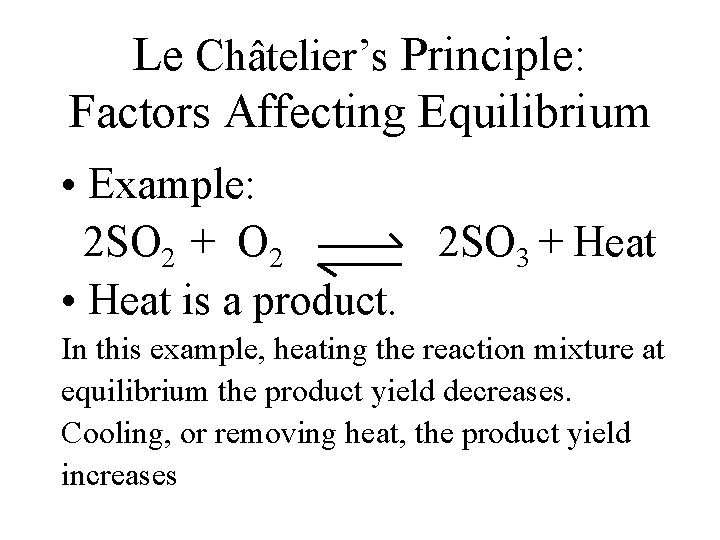
Le Châtelier’s Principle: Factors Affecting Equilibrium • Example: 2 SO 2 + O 2 2 SO 3 + Heat • Heat is a product. In this example, heating the reaction mixture at equilibrium the product yield decreases. Cooling, or removing heat, the product yield increases

Le Châtelier’s Principle: Factors Affecting Equilibrium 3. - Pressure A change in the pressure on a system affects only an equilibrium that has an unequal number of moles of gaseous reactants and products.
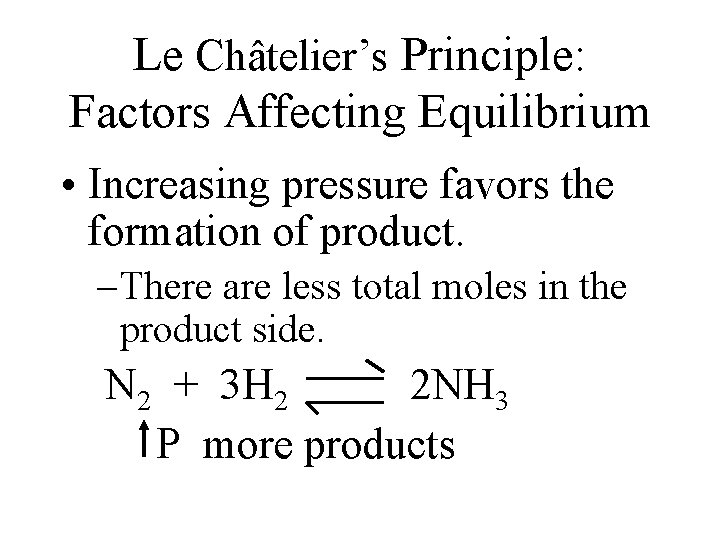
Le Châtelier’s Principle: Factors Affecting Equilibrium • Increasing pressure favors the formation of product. – There are less total moles in the product side. N 2 + 3 H 2 2 NH 3 P more products
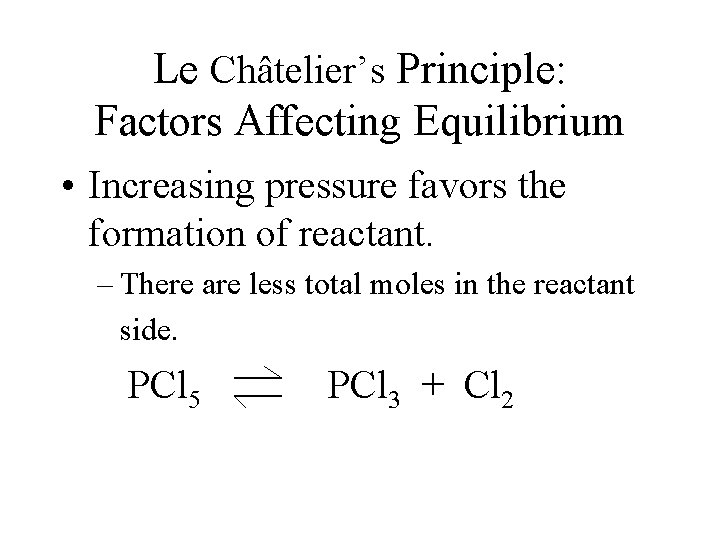
Le Châtelier’s Principle: Factors Affecting Equilibrium • Increasing pressure favors the formation of reactant. – There are less total moles in the reactant side. PCl 5 PCl 3 + Cl 2
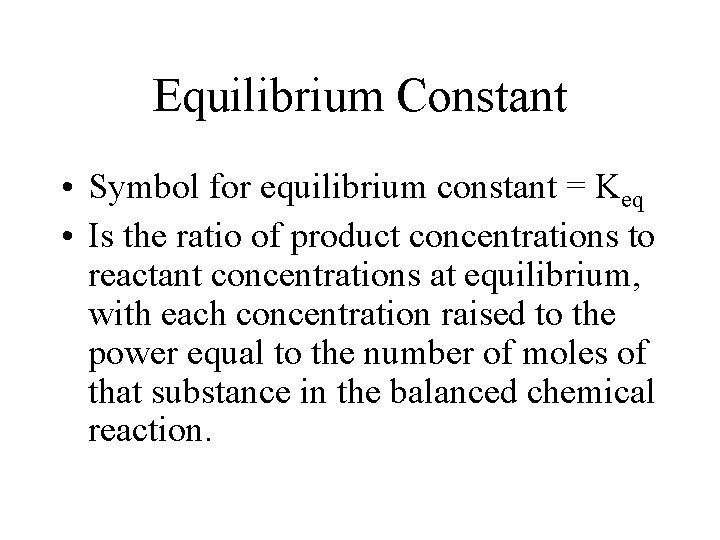
Equilibrium Constant • Symbol for equilibrium constant = Keq • Is the ratio of product concentrations to reactant concentrations at equilibrium, with each concentration raised to the power equal to the number of moles of that substance in the balanced chemical reaction.
![Equilibrium Constant • a. A + b. B c. C + d. D [C]c Equilibrium Constant • a. A + b. B c. C + d. D [C]c](http://slidetodoc.com/presentation_image_h2/45078e4c7ad77a3c571da481fa7a5b1a/image-14.jpg)
Equilibrium Constant • a. A + b. B c. C + d. D [C]c x [D]d • Keq = [A]a x [B]b
![Equilibrium Constant • The [ ] square brackets indicate the concentrations of substances in Equilibrium Constant • The [ ] square brackets indicate the concentrations of substances in](http://slidetodoc.com/presentation_image_h2/45078e4c7ad77a3c571da481fa7a5b1a/image-15.jpg)
Equilibrium Constant • The [ ] square brackets indicate the concentrations of substances in moles per liter (mol/L) • mol/L = Molarity • mol/L = M
![Equilibrium Constant 2 SO 2 + O 2 2 SO 3 2 [SO 3] Equilibrium Constant 2 SO 2 + O 2 2 SO 3 2 [SO 3]](http://slidetodoc.com/presentation_image_h2/45078e4c7ad77a3c571da481fa7a5b1a/image-16.jpg)
Equilibrium Constant 2 SO 2 + O 2 2 SO 3 2 [SO 3] Keq = [SO 2]2 [O 2]
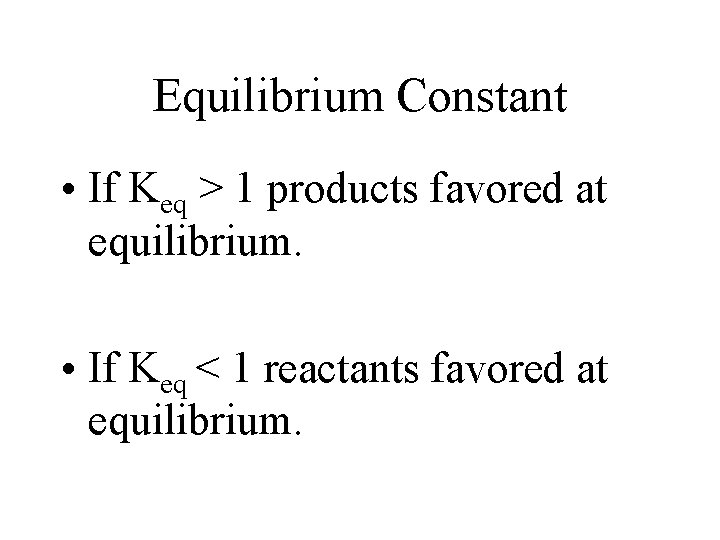
Equilibrium Constant • If Keq > 1 products favored at equilibrium. • If Keq < 1 reactants favored at equilibrium.
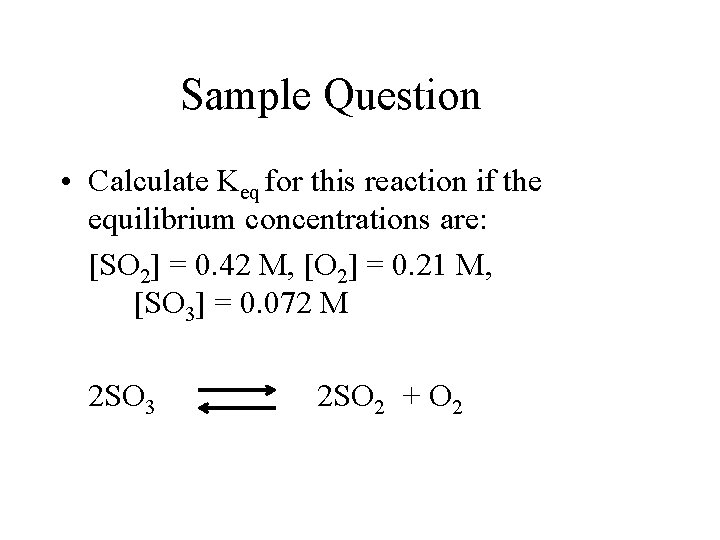
Sample Question • Calculate Keq for this reaction if the equilibrium concentrations are: [SO 2] = 0. 42 M, [O 2] = 0. 21 M, [SO 3] = 0. 072 M 2 SO 3 2 SO 2 + O 2
- Slides: 18