Equilibrium Chapter 18 Collision Theory a reaction is

Equilibrium Chapter 18

Collision Theory ¡ ¡ a reaction is more likely to occur if reactant particles collide with proper energy and orientation Reaction Rate l ¡ How fast the reaction proceeds Activation Energy (EA) l Minimum energy that colliding particles must have in order to react
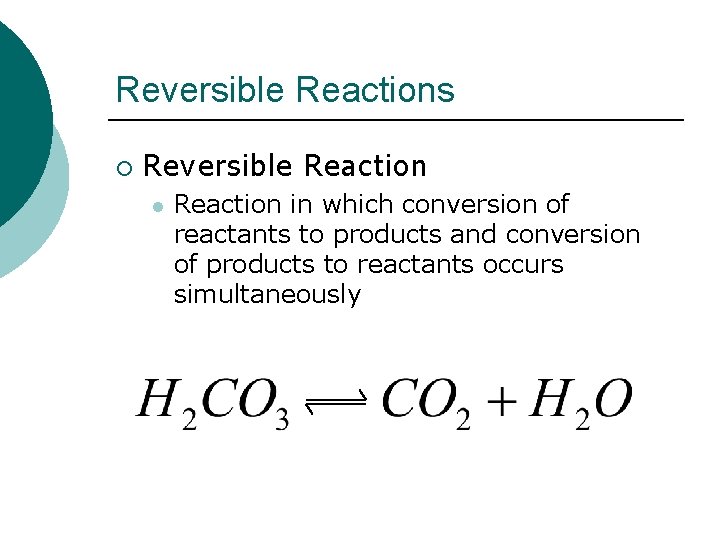
Reversible Reactions ¡ Reversible Reaction l Reaction in which conversion of reactants to products and conversion of products to reactants occurs simultaneously
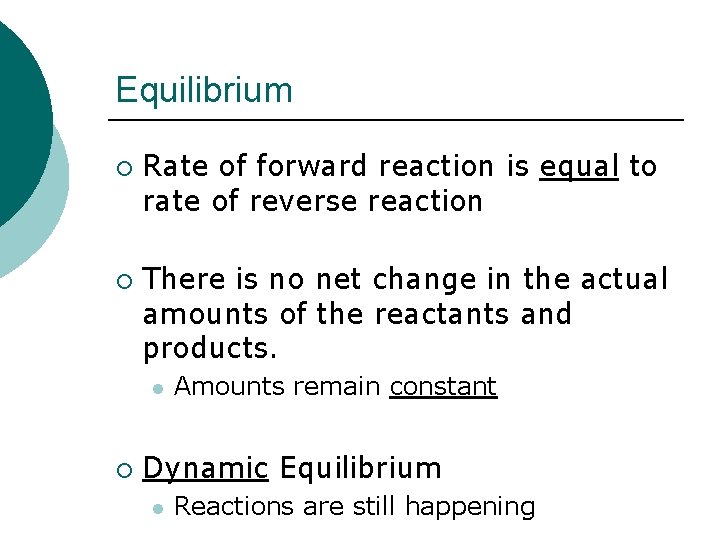
Equilibrium ¡ ¡ Rate of forward reaction is equal to rate of reverse reaction There is no net change in the actual amounts of the reactants and products. l ¡ Amounts remain constant Dynamic Equilibrium l Reactions are still happening

Equilibrium ¡ Saturated Solution l Solid in equilibrium with dissolved particles

Equilibrium Constants Temperature dependent

Le. Chatelier’s Principle ¡ If a stress is applied to a system in dynamic equilibrium, the system changes in a way that relieves the stress
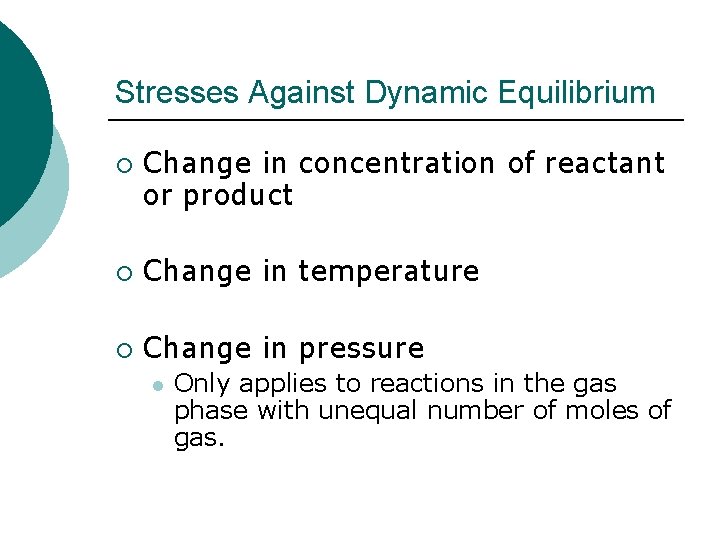
Stresses Against Dynamic Equilibrium ¡ Change in concentration of reactant or product ¡ Change in temperature ¡ Change in pressure l Only applies to reactions in the gas phase with unequal number of moles of gas.

Example ¡ ¡ How does adding more carbon dioxide shift equilibrium? Equilibrium will shift towards reactants

What does “Shifting” mean? Adding more products will cause more reverse reaction to occur ¡ More products are converted into reactants ¡ Rate of reverse reaction increases ¡ Amounts of products decrease, reactants increase ¡ Shift towards Reactants ¡
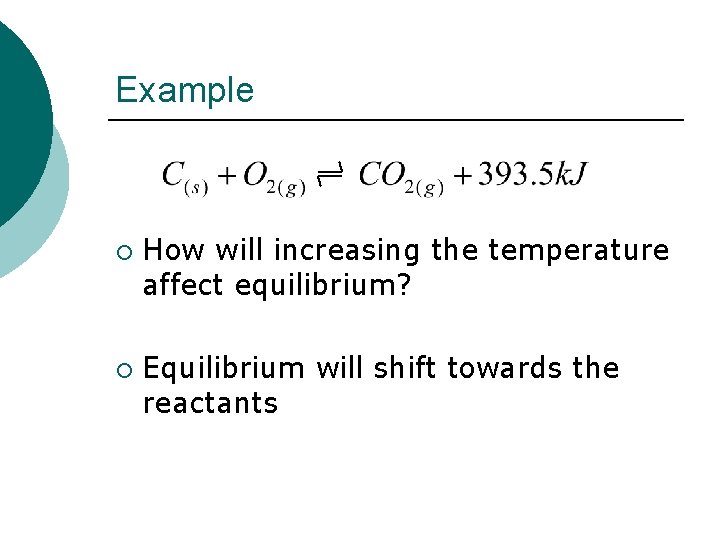
Example ¡ ¡ How will increasing the temperature affect equilibrium? Equilibrium will shift towards the reactants

Example ¡ How will increasing pressure affect equilibrium? ¡ Equilibrium will shift towards the products l Increasing pressure always shifts equilibrium towards the side with the least number of moles of gas.
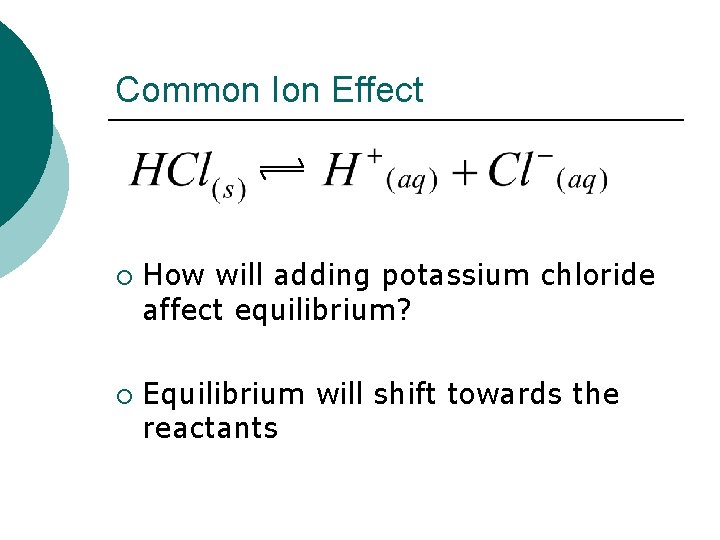
Common Ion Effect ¡ ¡ How will adding potassium chloride affect equilibrium? Equilibrium will shift towards the reactants


Review ¡ Endothermic l l ¡ Energy being added N 2 + O 2 + 182. 6 k. J 2 NO Exothermic l l Energy being released 2 CO + O 2 2 CO 2 + 566 k. J
Potential Energy ¡ Potential Energy (PE) l ¡ Energy stored in chemical bonds Heat of Reaction (ΔH) l l Energy absorbed or released during a chemical reaction PEProducts – PEReactants
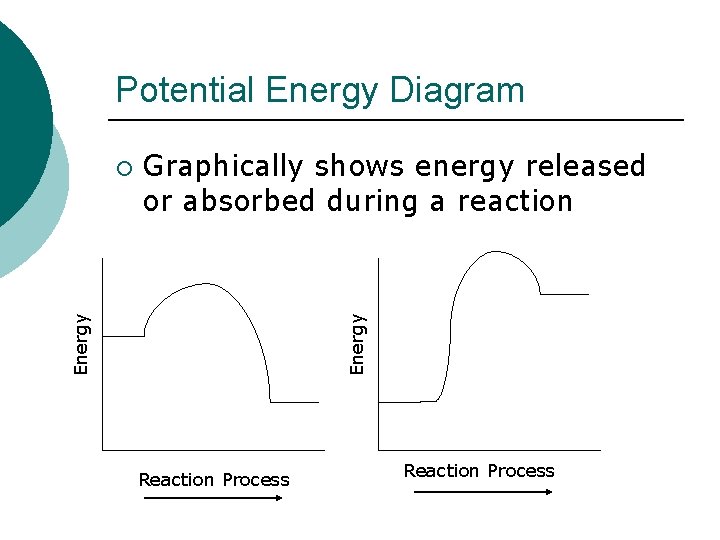
Potential Energy Diagram Graphically shows energy released or absorbed during a reaction Energy ¡ Reaction Process
Potential Energy Diagram ¡ On a Potential Energy Diagram, you must be able to identify the following: l l Potential Energy of Reactants, PEReactants Potential Energy of Products, PEProducts Heat of Reaction, ΔH Activation Energy, EA
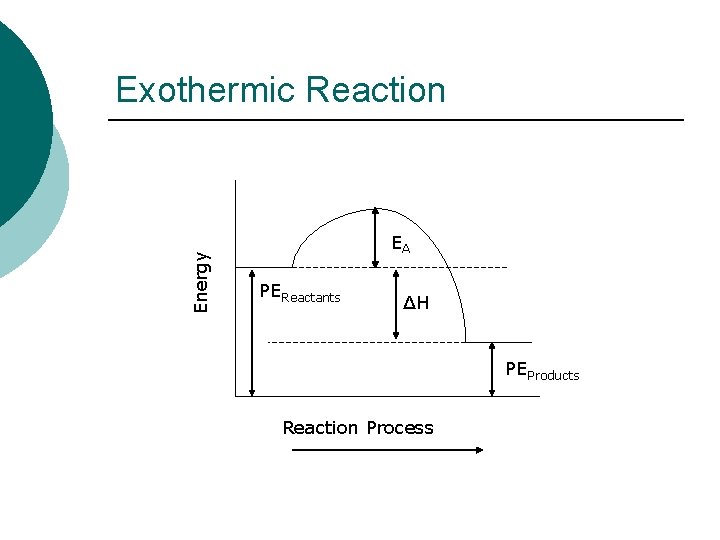
Energy Exothermic Reaction EA PEReactants ΔH PEProducts Reaction Process
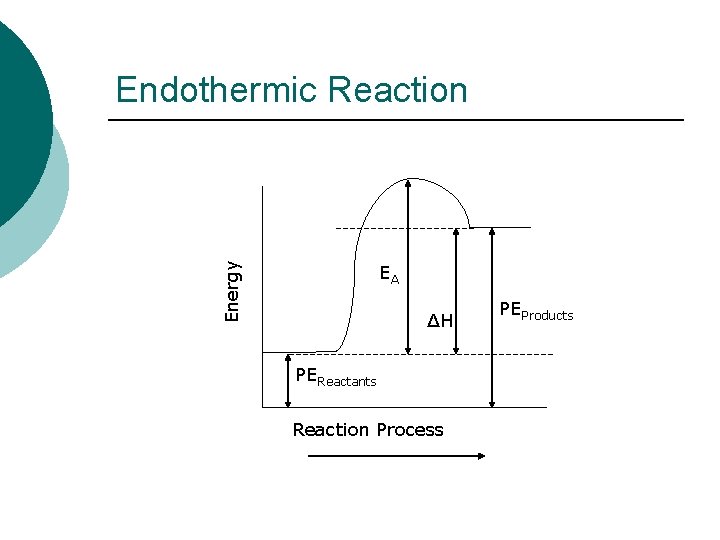
Energy Endothermic Reaction EA ΔH PEReactants Reaction Process PEProducts
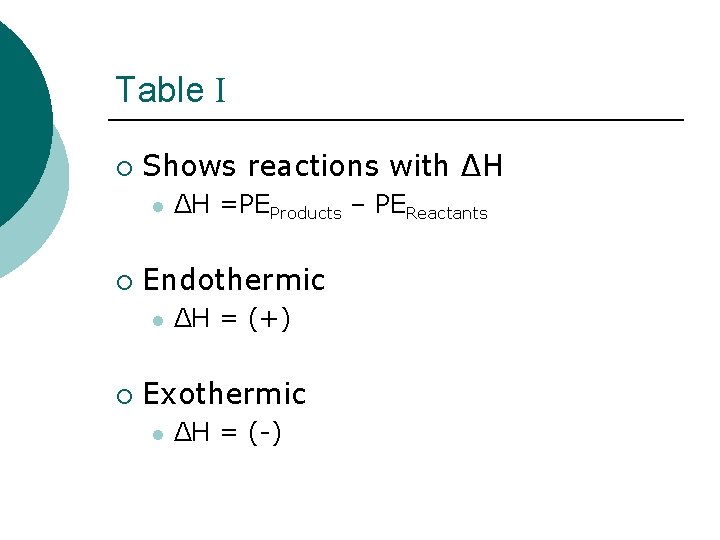
Table I ¡ Shows reactions with ΔH l ¡ Endothermic l ¡ ΔH =PEProducts – PEReactants ΔH = (+) Exothermic l ΔH = (-)

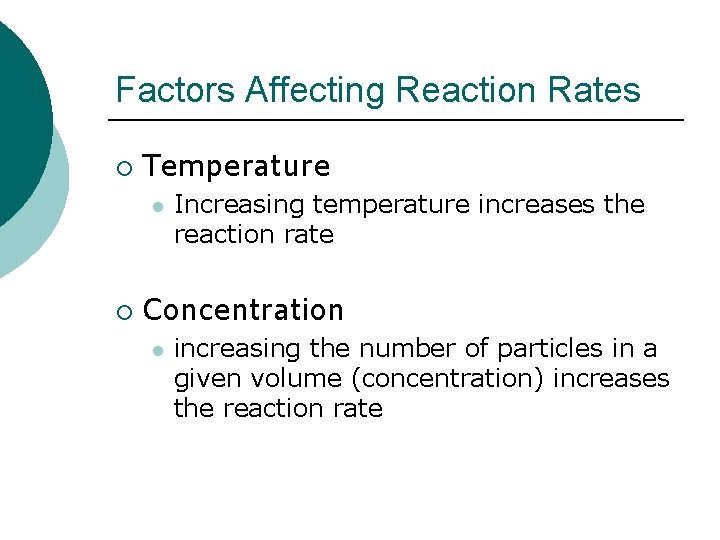
Factors Affecting Reaction Rates ¡ Temperature l ¡ Increasing temperature increases the reaction rate Concentration l increasing the number of particles in a given volume (concentration) increases the reaction rate
Factors Affecting Reaction Rates ¡ Surface Area l ¡ increasing surface area increases reaction rate Catalyst l l the presence of a catalyst will often increase reaction rate Catalysts are not used up during a reaction
Affect of a Catalyst ¡ Provides an alternate pathway for the reaction to proceed ¡ Decreases activation energy ¡ Increases reaction rate
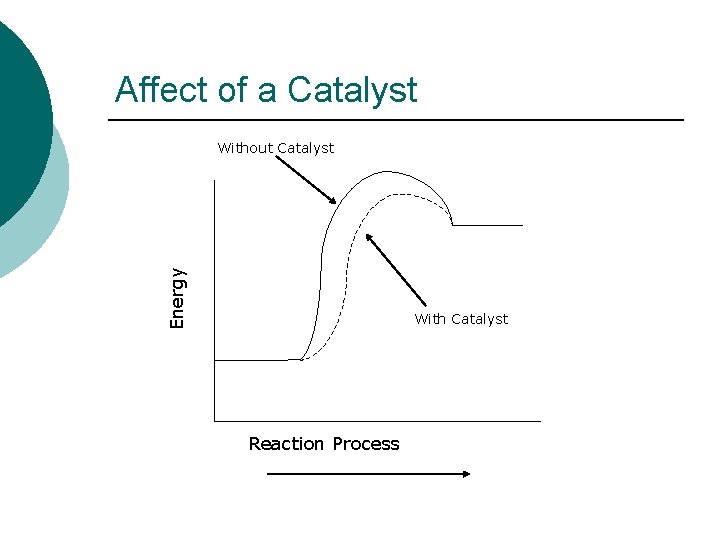
Affect of a Catalyst Energy Without Catalyst With Catalyst Reaction Process
Entropy ¡ ¡ Measure of randomness or disorder Systems in nature tend to undergo changes towards lower energy and higher entropy l The universe is lazy and disorganized

Entropy ¡ Increasing Entropy l l Solid Liquid Gas Solid Dissolved
- Slides: 28