Equilibrium Chapter 13 Dynamic Equilibrium n Exists in

Equilibrium Chapter 13
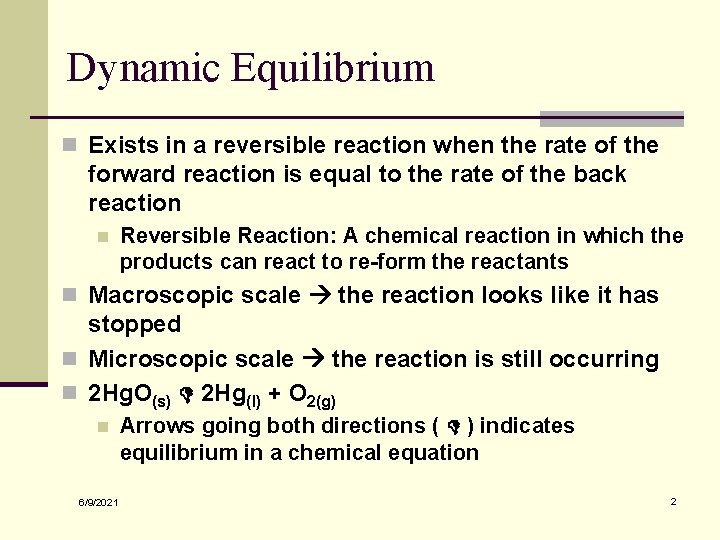
Dynamic Equilibrium n Exists in a reversible reaction when the rate of the forward reaction is equal to the rate of the back reaction n Reversible Reaction: A chemical reaction in which the products can react to re-form the reactants n Macroscopic scale the reaction looks like it has stopped n Microscopic scale the reaction is still occurring n 2 Hg. O(s) 2 Hg(l) + O 2(g) n 6/9/2021 Arrows going both directions ( ) indicates equilibrium in a chemical equation 2
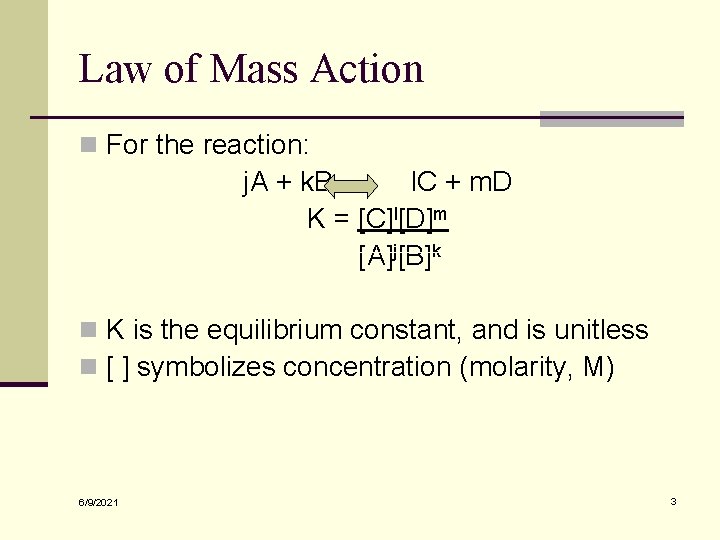
Law of Mass Action n For the reaction: j. A + k. B l. C + m. D K = [C]l[D]m [A]j[B]k n K is the equilibrium constant, and is unitless n [ ] symbolizes concentration (molarity, M) 6/9/2021 3
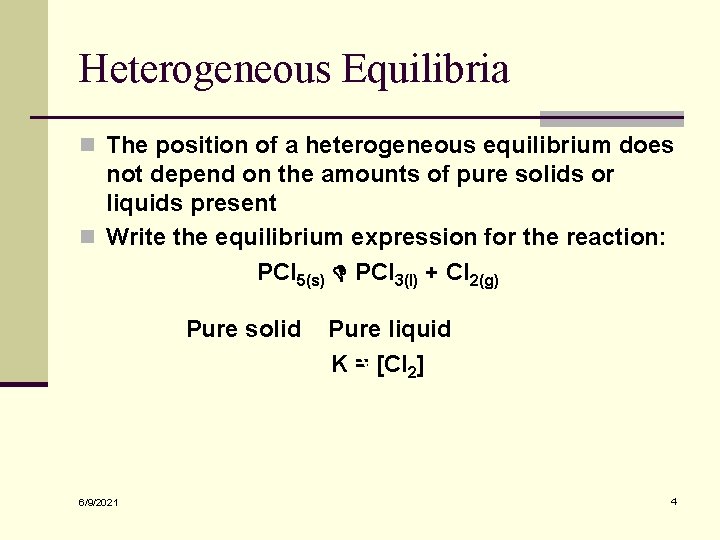
Heterogeneous Equilibria n The position of a heterogeneous equilibrium does not depend on the amounts of pure solids or liquids present n Write the equilibrium expression for the reaction: PCl 5(s) PCl 3(l) + Cl 2(g) Pure solid 6/9/2021 Pure liquid K = [Cl 2] 4

Product Favored Equilibrium n Large values for K signify the reaction is “product favored” n When equilibrium is achieved, most reactant has been converted to product 6/9/2021 5

Reactant Favored Equilibrium n Small values for K signify the reaction is “reactant favored” n When equilibrium is achieved, very little reactant has been converted to product 6/9/2021 6
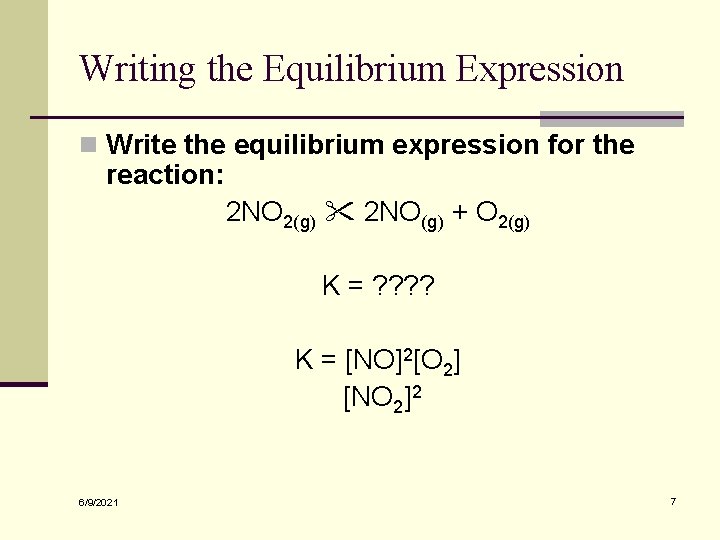
Writing the Equilibrium Expression n Write the equilibrium expression for the reaction: 2 NO 2(g) 2 NO(g) + O 2(g) K = ? ? K = [NO]2[O 2] [NO 2]2 6/9/2021 7
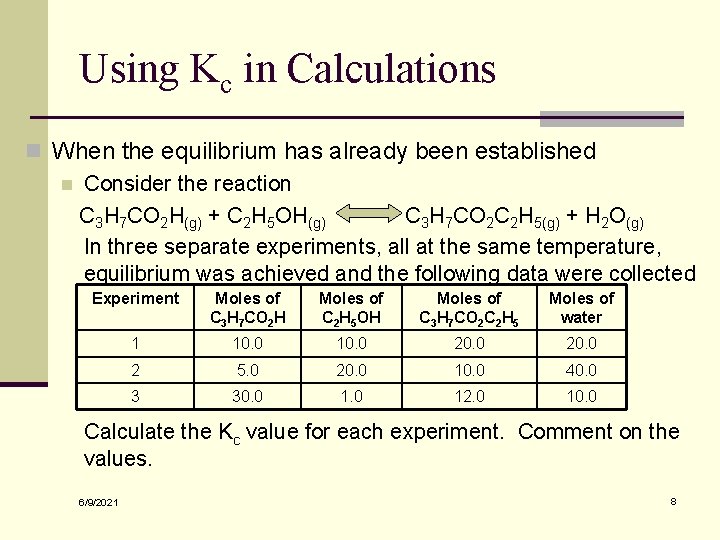
Using Kc in Calculations n When the equilibrium has already been established n Consider the reaction C 3 H 7 CO 2 H(g) + C 2 H 5 OH(g) C 3 H 7 CO 2 C 2 H 5(g) + H 2 O(g) In three separate experiments, all at the same temperature, equilibrium was achieved and the following data were collected Experiment Moles of C 3 H 7 CO 2 H Moles of C 2 H 5 OH Moles of C 3 H 7 CO 2 C 2 H 5 Moles of water 1 10. 0 20. 0 2 5. 0 20. 0 10. 0 40. 0 3 30. 0 12. 0 10. 0 Calculate the Kc value for each experiment. Comment on the values. 6/9/2021 8
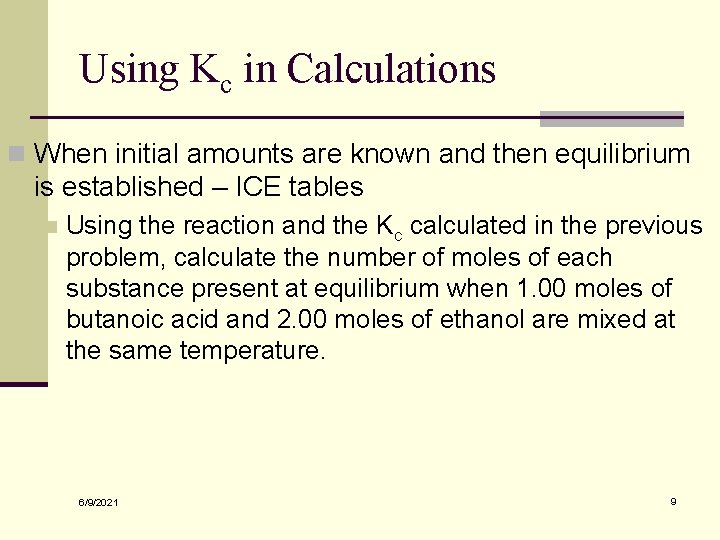
Using Kc in Calculations n When initial amounts are known and then equilibrium is established – ICE tables n Using the reaction and the Kc calculated in the previous problem, calculate the number of moles of each substance present at equilibrium when 1. 00 moles of butanoic acid and 2. 00 moles of ethanol are mixed at the same temperature. 6/9/2021 9
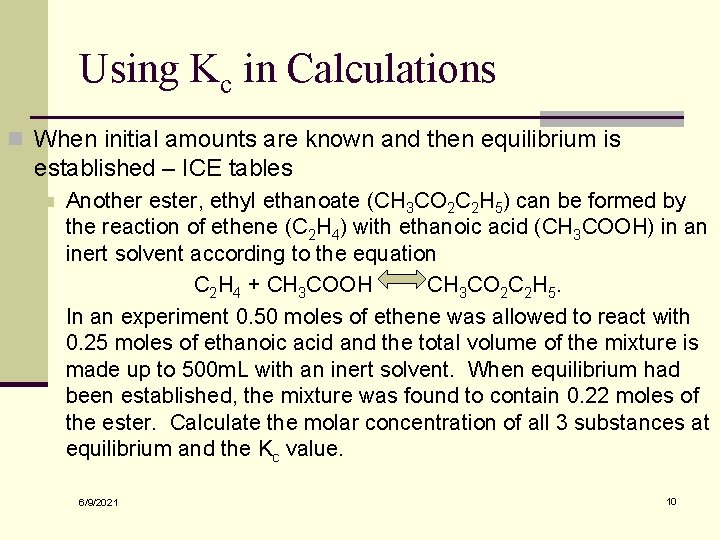
Using Kc in Calculations n When initial amounts are known and then equilibrium is established – ICE tables n Another ester, ethyl ethanoate (CH 3 CO 2 C 2 H 5) can be formed by the reaction of ethene (C 2 H 4) with ethanoic acid (CH 3 COOH) in an inert solvent according to the equation C 2 H 4 + CH 3 COOH CH 3 CO 2 C 2 H 5. In an experiment 0. 50 moles of ethene was allowed to react with 0. 25 moles of ethanoic acid and the total volume of the mixture is made up to 500 m. L with an inert solvent. When equilibrium had been established, the mixture was found to contain 0. 22 moles of the ester. Calculate the molar concentration of all 3 substances at equilibrium and the Kc value. 6/9/2021 10
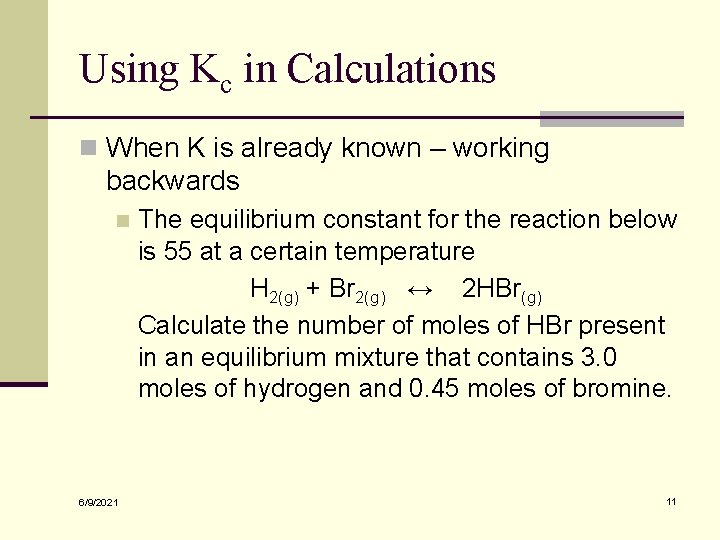
Using Kc in Calculations n When K is already known – working backwards n 6/9/2021 The equilibrium constant for the reaction below is 55 at a certain temperature H 2(g) + Br 2(g) ↔ 2 HBr(g) Calculate the number of moles of HBr present in an equilibrium mixture that contains 3. 0 moles of hydrogen and 0. 45 moles of bromine. 11
Le. Chatelier’s Principle n When a system at equilibrium is placed under stress, the system will undergo a change in such a way as to relieve that stress. n Translated: The system undergoes a temporary shift in order to restore equilibrium. 6/9/2021 12
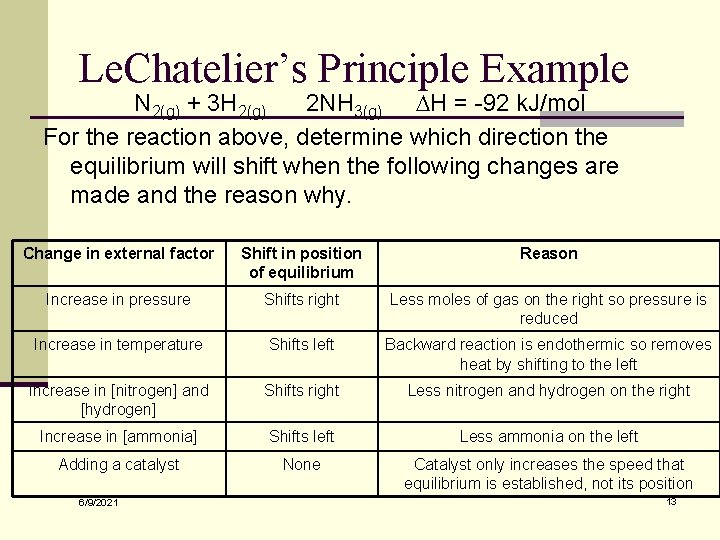
Le. Chatelier’s Principle Example N 2(g) + 3 H 2(g) 2 NH 3(g) DH = -92 k. J/mol For the reaction above, determine which direction the equilibrium will shift when the following changes are made and the reason why. Change in external factor Shift in position of equilibrium Reason Increase in pressure Shifts right Less moles of gas on the right so pressure is reduced Increase in temperature Shifts left Backward reaction is endothermic so removes heat by shifting to the left Increase in [nitrogen] and [hydrogen] Shifts right Less nitrogen and hydrogen on the right Increase in [ammonia] Shifts left Less ammonia on the left Adding a catalyst None Catalyst only increases the speed that equilibrium is established, not its position 6/9/2021 13
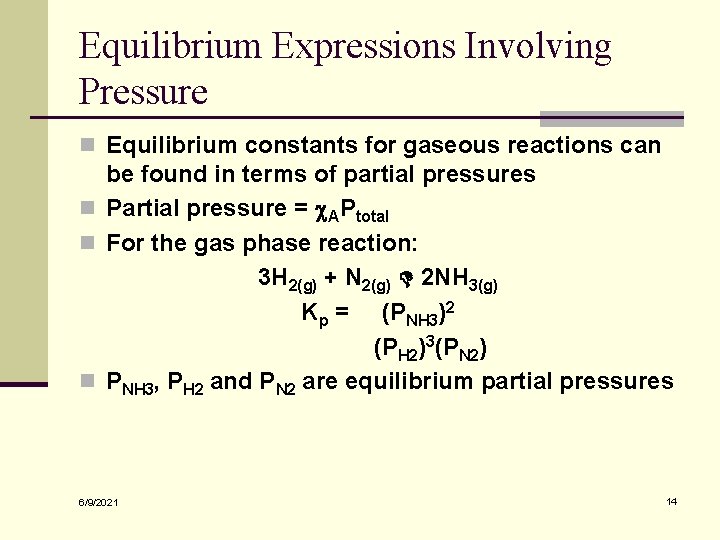
Equilibrium Expressions Involving Pressure n Equilibrium constants for gaseous reactions can be found in terms of partial pressures n Partial pressure = c. APtotal n For the gas phase reaction: 3 H 2(g) + N 2(g) 2 NH 3(g) Kp = (PNH 3)2 (PH 2)3(PN 2) n PNH 3, PH 2 and PN 2 are equilibrium partial pressures 6/9/2021 14

Use of Kp in calculations n Dinitrogen tetroxide dissociates into nitrogen dioxide according the to equation below N 2 O 4(g) 2 NO 2(g) When one mole of N 2 O 4, at an equilibrium pressure of 3. 0 atm is 45% dissociated, what is the value of Kp? n What will be the % dissociation of nitrogen tetroxide at the same temperature but at a total pressure of 1. 50 atm? n 6/9/2021 15
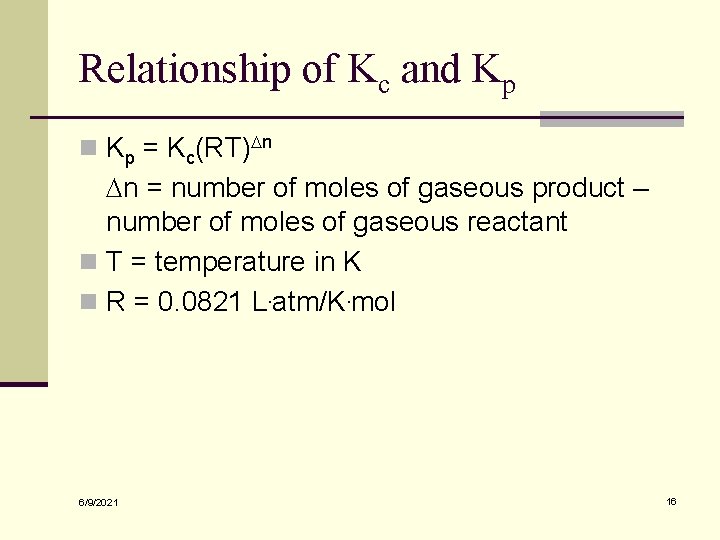
Relationship of Kc and Kp n Kp = Kc(RT)Dn · Dn = number of moles of gaseous product – number of moles of gaseous reactant n T = temperature in K n R = 0. 0821 L. atm/K. mol 6/9/2021 16

Format of Kc n When a reversible reaction is written in the opposite direction the new equilibrium constant is equal to the reciprocal of the original n If a reaction is “halved” or “doubled” then the original Kc will need to be square rooted or squared respectively to find the new Kc value 6/9/2021 17
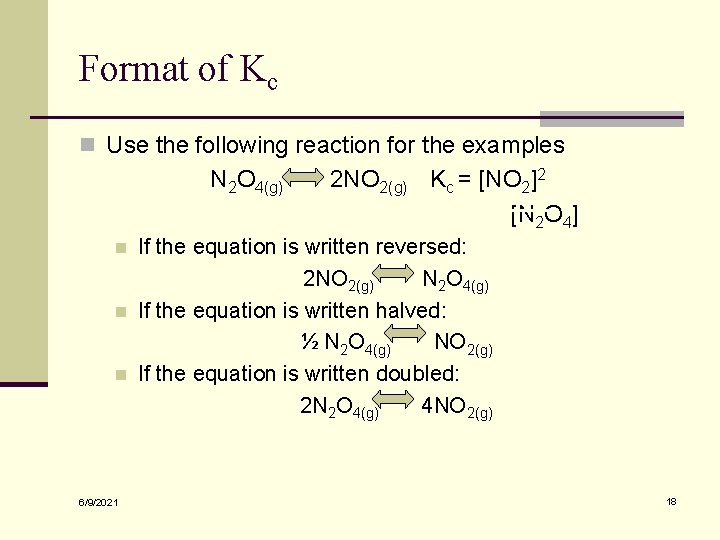
Format of Kc n Use the following reaction for the examples N 2 O 4(g) n n n 6/9/2021 2 NO 2(g) Kc = [NO 2]2 [N 2 O 4] If the equation is written reversed: 2 NO 2(g) N 2 O 4(g) If the equation is written halved: ½ N 2 O 4(g) NO 2(g) If the equation is written doubled: 2 N 2 O 4(g) 4 NO 2(g) 18
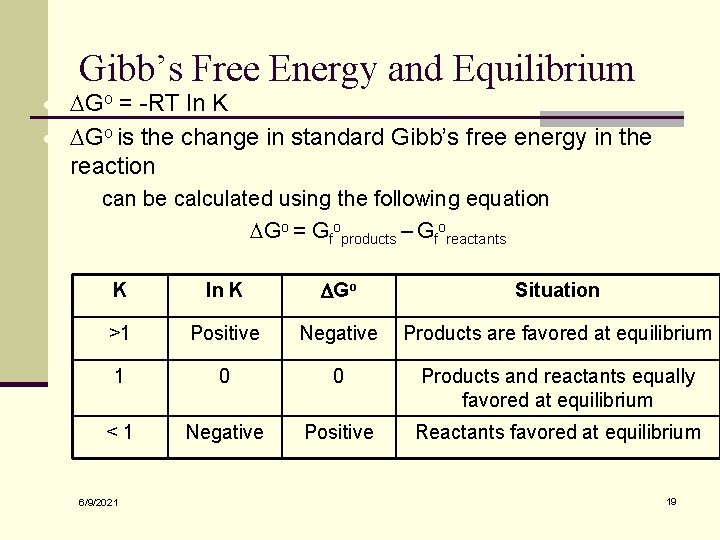
Gibb’s Free Energy and Equilibrium · DGo = -RT ln K · DGo is the change in standard Gibb’s free energy in the reaction · can be calculated using the following equation DGo = Gfoproducts – Gforeactants K ln K DGo Situation >1 Positive Negative Products are favored at equilibrium 1 0 0 Products and reactants equally favored at equilibrium <1 Negative Positive Reactants favored at equilibrium 6/9/2021 19
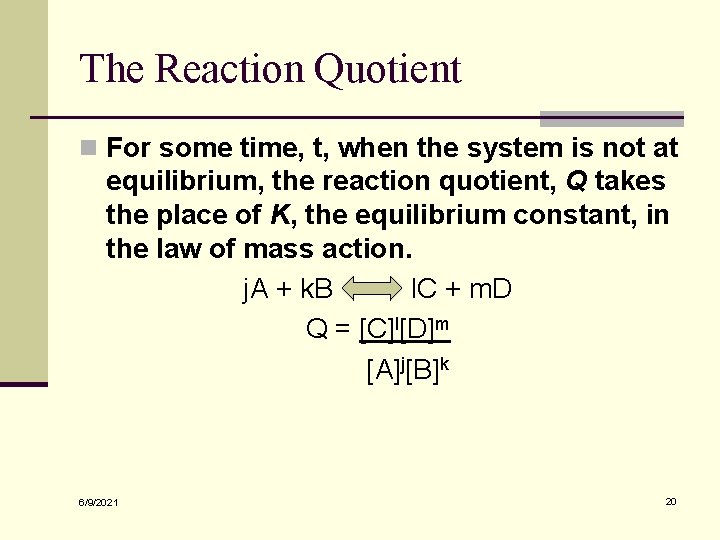
The Reaction Quotient n For some time, t, when the system is not at equilibrium, the reaction quotient, Q takes the place of K, the equilibrium constant, in the law of mass action. j. A + k. B l. C + m. D Q = [C]l[D]m [A]j[B]k 6/9/2021 20
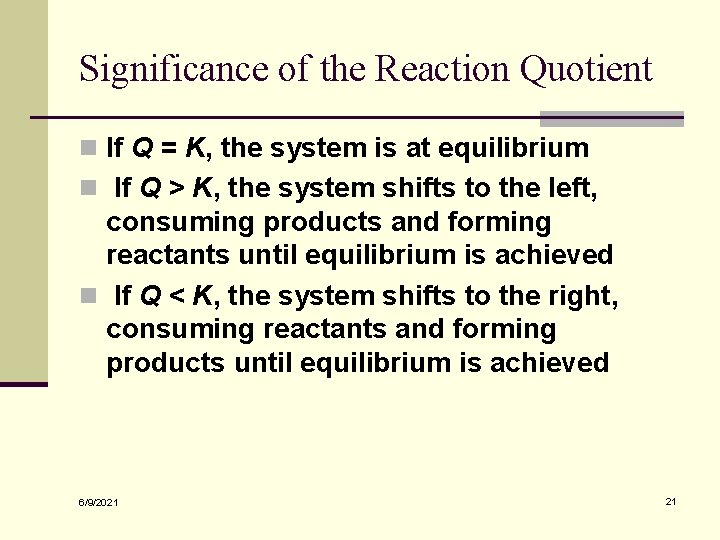
Significance of the Reaction Quotient n If Q = K, the system is at equilibrium n If Q > K, the system shifts to the left, consuming products and forming reactants until equilibrium is achieved n If Q < K, the system shifts to the right, consuming reactants and forming products until equilibrium is achieved 6/9/2021 21

Solubility Product, Ksp n Most salts can dissolve (dissociate) to some degree, even the ones considered insoluble n Ksp deals with this dissociation n Ksp is defined as the product of the molar concentrations of the constituent ions raised to their stoichiometric coefficients 6/9/2021 22
Solubility Product, Ksp n The smaller the Ksp, the smaller the number of ions in the solution and therefore the less soluble the compound is n Use Q when equilibrium has not been established to determine if a precipitate will form If Q > Ksp a precipitate will form n If Q < Ksp no precipitate will form (the solution is saturated) n 6/9/2021 23

Solubility Product, Ksp n Other ways of expressing the amount of solute dissolved in a solvent Molar solubility – the number of moles of a solute in 1 L of a saturated solution n Solubility – the number of grams of a solute in 1 L of a saturated solution n 6/9/2021 24
Solubility Product, Ksp n Examples n At a certain temperature the molar solubility of the salt M 2+X 2 - is found to be 4. 15 x 10 -6 M. Calculate the Ksp for the salt n At a certain temperature, the solubility of calcium sulfate is found to be 0. 66 g/L. Calculate the Ksp for calcium sulfate. n Given that Ksp for Ag 2 SO 4 = 1. 41 x 10 -5, calculate the solubility and molar solubility of Ag 2 SO 4. n If 150. m. L of 0. 00395 M Ba. Cl 2 and 550. m. L of 0. 00775 M K 2 SO 4 are mixed, will a precipitate form? (Ksp for barium sulfate is 1. 1 x 10 -10) 6/9/2021 25

Common Ion Effect n If a solution contains two dissolved substances that share a common ion, then the solubility of a salt becomes more complex n Example n 6/9/2021 Given the Ksp for silver chloride as 1. 58 x 10 -10, calculate the solubility of silver chloride in n Pure water n A solution that has a silver ion concentration of 5. 5 10 3 M n A 0. 025 M KCl solution 26
- Slides: 26