Equilibrium and NonEquilibrium Thermodynamics of Natural Gas Processing

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Measurement and Modelling of Absorption of Carbon Dioxide into Methyldiethanolamine Solutions at High Pressures Ph. D Dissertation Even Solbraa 14. February 2003

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing What has been done ? • A high pressure experimental equipment has been built and new high pressure experimental data are presented • Equilibrium and kinetic limitations related to CO 2 removal at high pressures in MDEA solutions are identified • Neq. Sim - a general simulation program for natural gas processing operations has been developed. It is based on equilibrium and non-equilibrium models developed in this work. Many types of processes can now be solved effectively using a general non equilibrium two-fluid model

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing How are the results used today? • Capacity and kinetic limits of high pressure absorption processes of CO 2 in MDEA-solutions are estimated • The simulation program developed is used to solve and teach thermodynamics and mass transfer processes • High pressure equilibrium (e. g. dew point) and non-equilibrium (e. g. drying) processes are solved in an effective way

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Outline 1. Introduction to Natural Gas Processing and Transport 2. Equilibrium and Non-Equilibrium Model Development 3. Presentation of the Simulation Program Developed 4. Modelling and Regression to Experimental Data 5. Experimental Work and Results 6. Conclusions
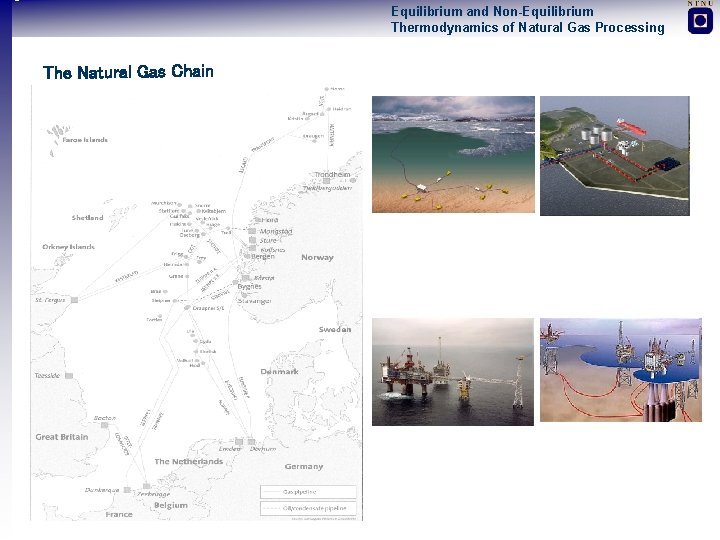
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing The Natural Gas Chain
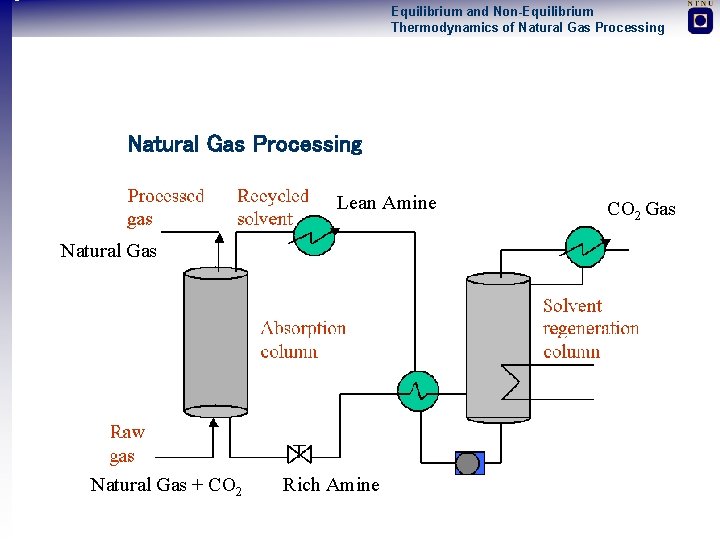
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Lean Amine Natural Gas + CO 2 Rich Amine CO 2 Gas
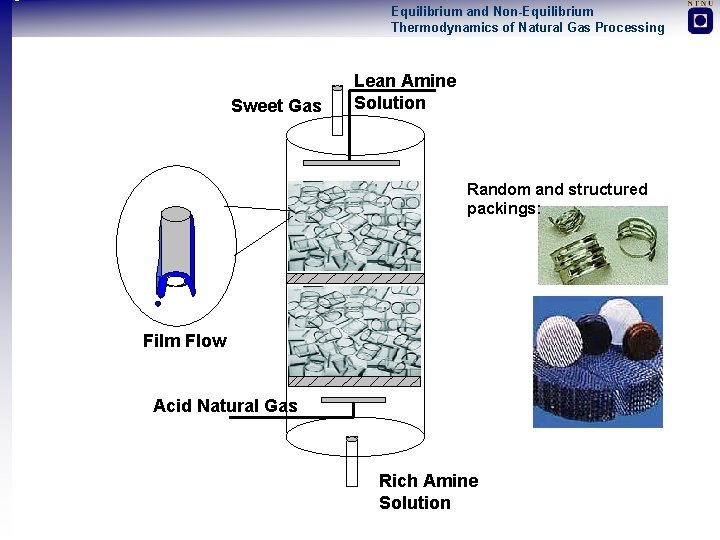
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Sweet Gas Lean Amine Solution Random and structured packings: Film Flow Acid Natural Gas Rich Amine Solution
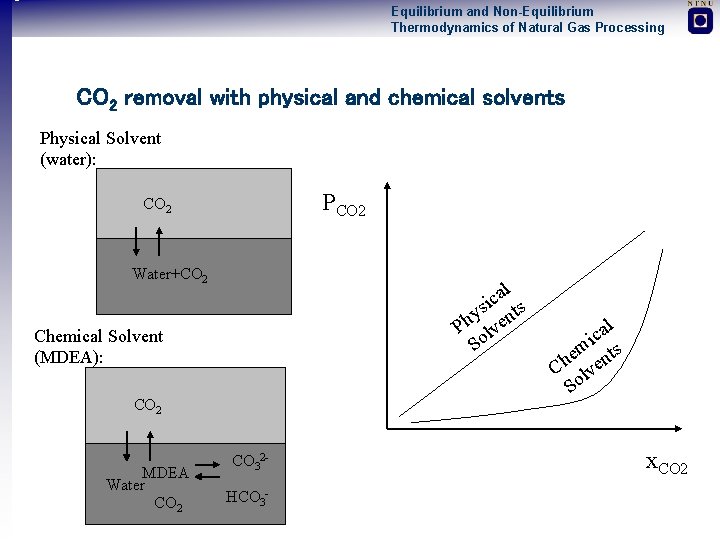
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing CO 2 removal with physical and chemical solvents Physical Solvent (water): PCO 2 Water+CO 2 l a ic ts s y Ph lven So Chemical Solvent (MDEA): CO 2 MDEA Water CO 2 CO 32 HCO 3 - l a ic m e nts h C lve So x. CO 2
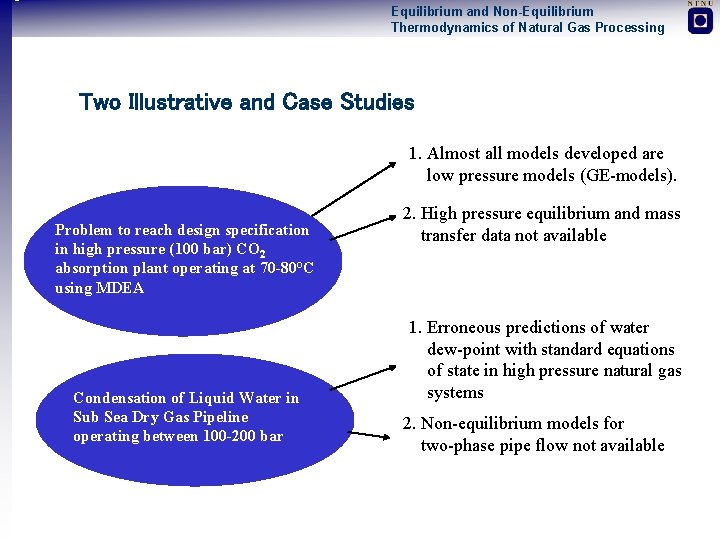
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Two Illustrative and Case Studies 1. Almost all models developed are low pressure models (GE-models). Problem to reach design specification in high pressure (100 bar) CO 2 absorption plant operating at 70 -80°C using MDEA Condensation of Liquid Water in Sub Sea Dry Gas Pipeline operating between 100 -200 bar 2. High pressure equilibrium and mass transfer data not available 1. Erroneous predictions of water dew-point with standard equations of state in high pressure natural gas systems 2. Non-equilibrium models for two-phase pipe flow not available

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Outline 1. Introduction to Natural Gas Processing and Transport 2. Equilibrium and Non-Equilibrium Model Development 3. Presentation of the Simulation Program Developed 4. Modelling and Regression to Experimental Data 5. Experimental Work and Results 6. Conclusions
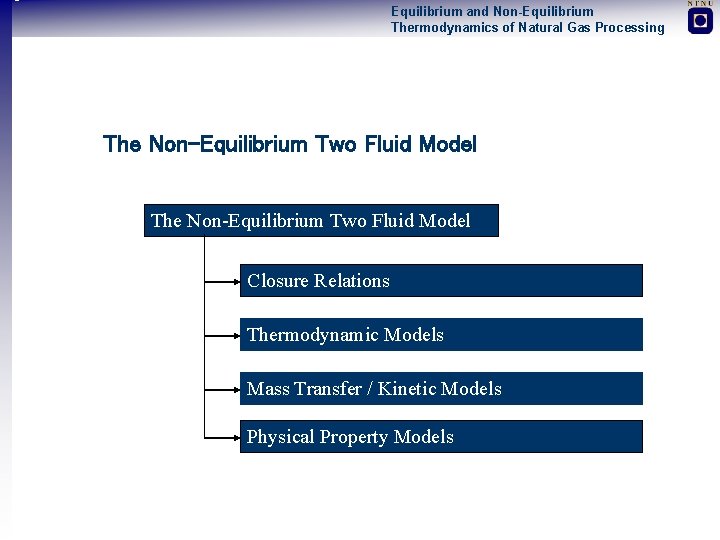
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing The Non-Equilibrium Two Fluid Model Closure Relations Thermodynamic Models Mass Transfer / Kinetic Models Physical Property Models
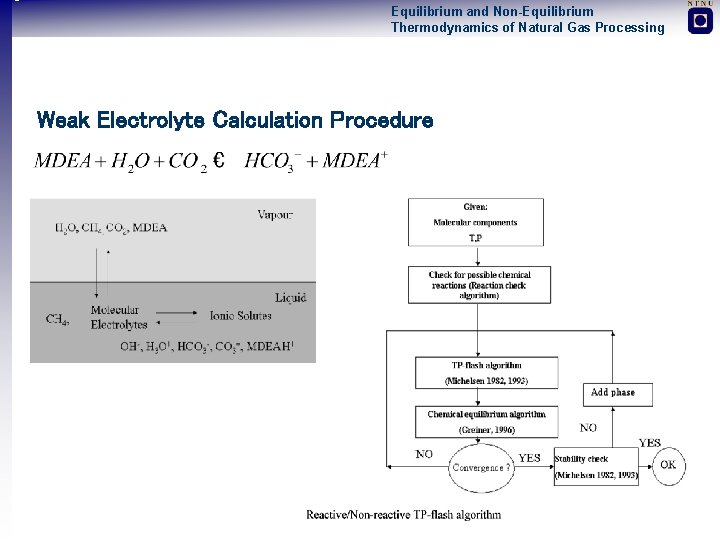
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Weak Electrolyte Calculation Procedure
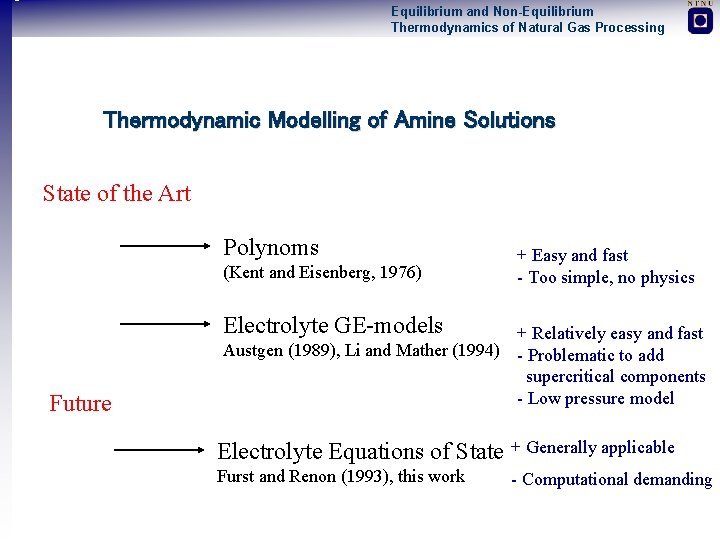
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Thermodynamic Modelling of Amine Solutions State of the Art Polynoms (Kent and Eisenberg, 1976) + Easy and fast - Too simple, no physics Electrolyte GE-models Future + Relatively easy and fast Austgen (1989), Li and Mather (1994) - Problematic to add supercritical components - Low pressure model Electrolyte Equations of State + Generally applicable Furst and Renon (1993), this work - Computational demanding
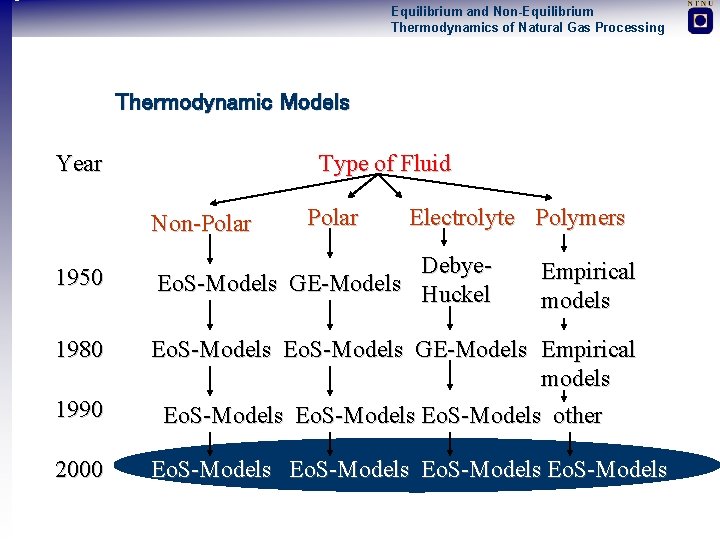
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Thermodynamic Models Year Type of Fluid Non-Polar Electrolyte Polymers 1950 Debye Eo. S-Models GE-Models Huckel 1980 Eo. S-Models GE-Models Empirical models 1990 2000 Empirical models Eo. S-Models other Eo. S-Models
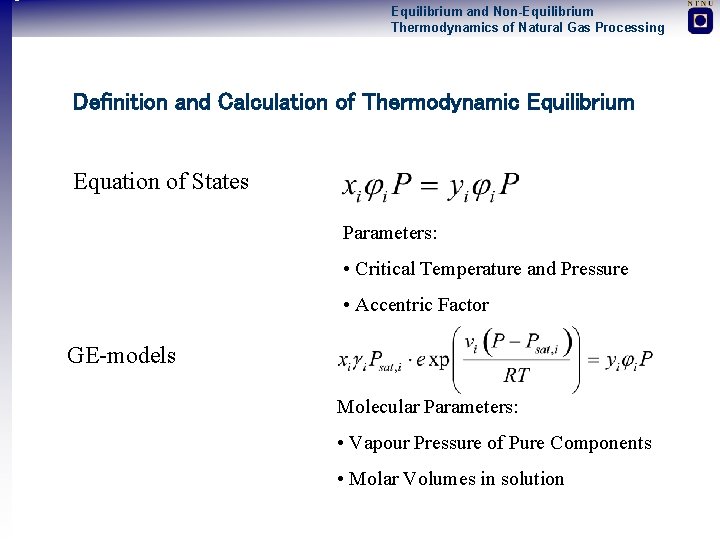
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Definition and Calculation of Thermodynamic Equilibrium Equation of States Parameters: • Critical Temperature and Pressure • Accentric Factor GE-models Molecular Parameters: • Vapour Pressure of Pure Components • Molar Volumes in solution
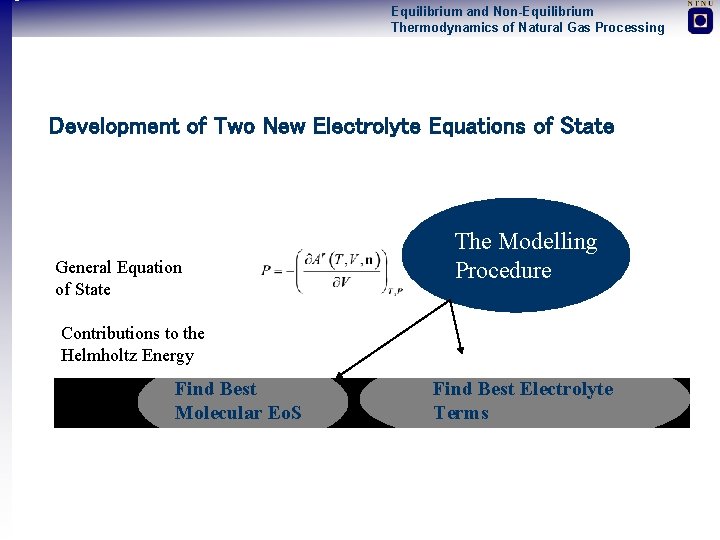
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Development of Two New Electrolyte Equations of State General Equation of State The Modelling Procedure Contributions to the Helmholtz Energy Find Best Molecular Eo. S Find Best Electrolyte Terms
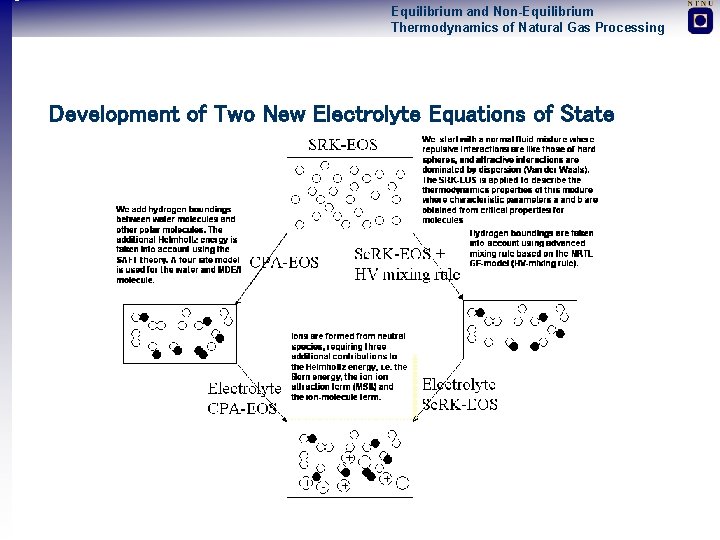
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Development of Two New Electrolyte Equations of State

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Equations of State Considered Equations of State Mixing Rules • RK (Redlich Kwong, 1949) • no • SRK (Soave, 1971) • Classic (Van der Waals, 1905) • PR (Peng and Robinson, 1979) • Huron Vidal (Huron-Vidal, 1979) • Sc. RK (Scwartzentruber and Renon, 1989) • Wong-Sandler (Wong and Sandler, 1993) • CPA (Kontegorgios, 1999) Electrolyte Extensions • Debye-Huckel (Debye-Huckel, 1952) • MSA (Blum and Høye, 1982) • Furst and Renon (Furst and Renon, 1993)
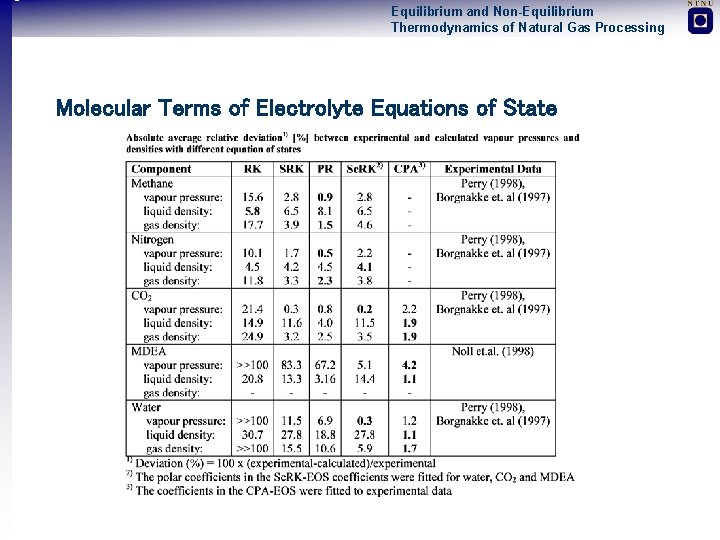
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Molecular Terms of Electrolyte Equations of State
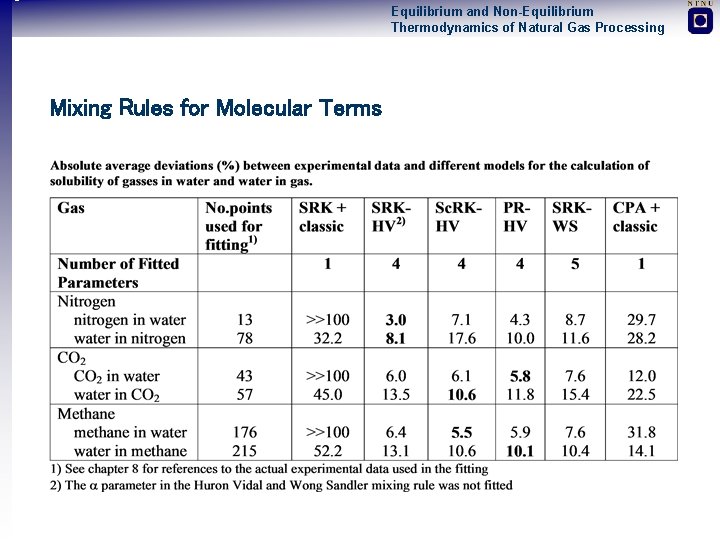
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Mixing Rules for Molecular Terms
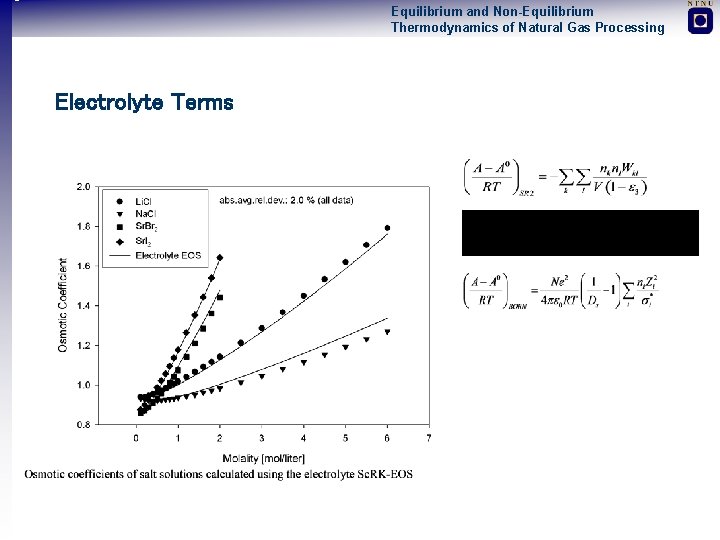
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Electrolyte Terms
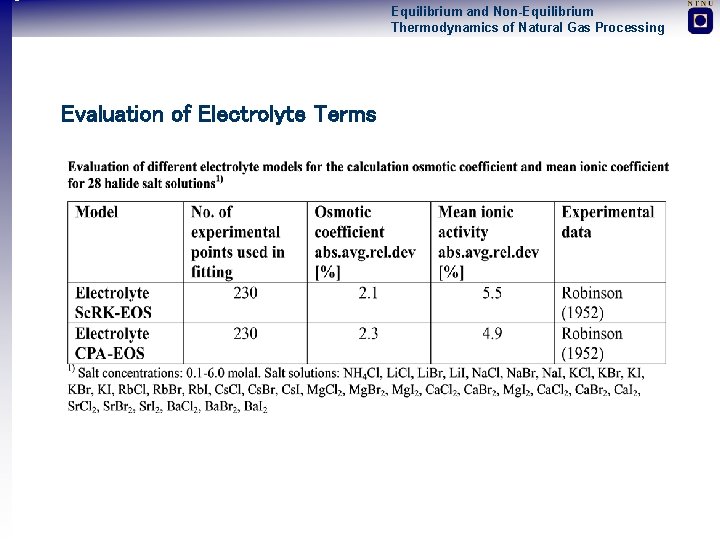
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Evaluation of Electrolyte Terms
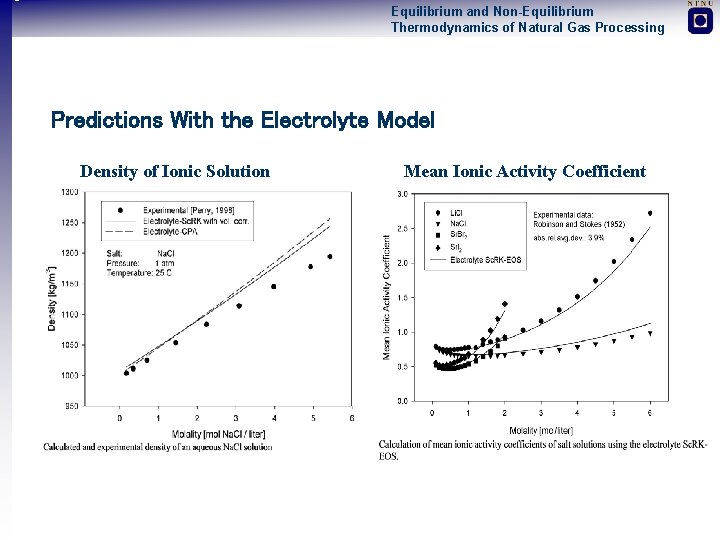
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Predictions With the Electrolyte Model Density of Ionic Solution Mean Ionic Activity Coefficient
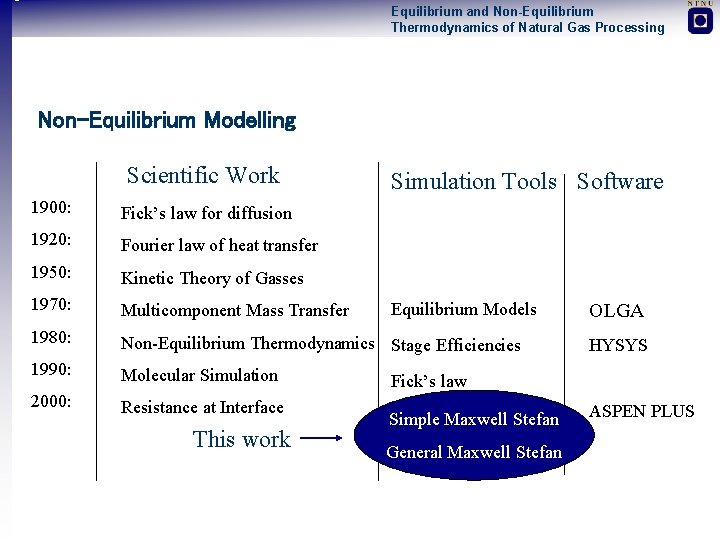
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Non-Equilibrium Modelling Scientific Work Simulation Tools Software 1900: Fick’s law for diffusion 1920: Fourier law of heat transfer 1950: Kinetic Theory of Gasses 1970: Multicomponent Mass Transfer 1980: Non-Equilibrium Thermodynamics Stage Efficiencies 1990: Molecular Simulation 2000: Resistance at Interface This work Equilibrium Models OLGA HYSYS Fick’s law Simple Maxwell Stefan General Maxwell Stefan ASPEN PLUS
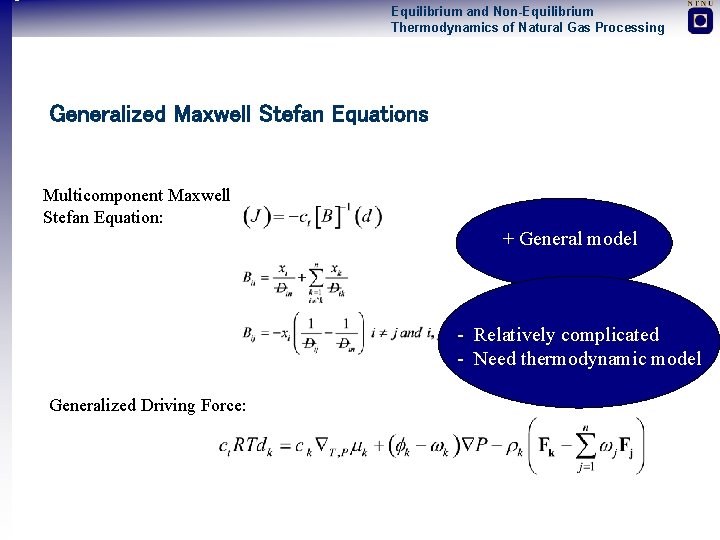
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Generalized Maxwell Stefan Equations Multicomponent Maxwell Stefan Equation: + General model - Relatively complicated - Need thermodynamic model Generalized Driving Force:
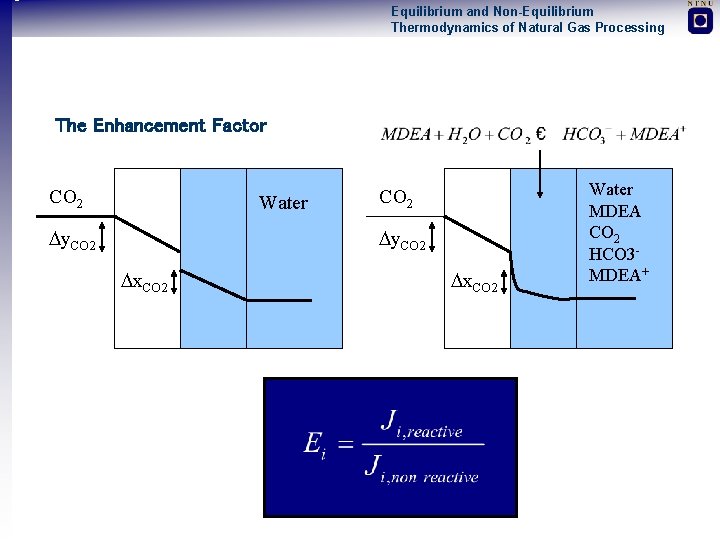
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing The Enhancement Factor CO 2 Water y. CO 2 x. CO 2 Water MDEA CO 2 HCO 3 MDEA+
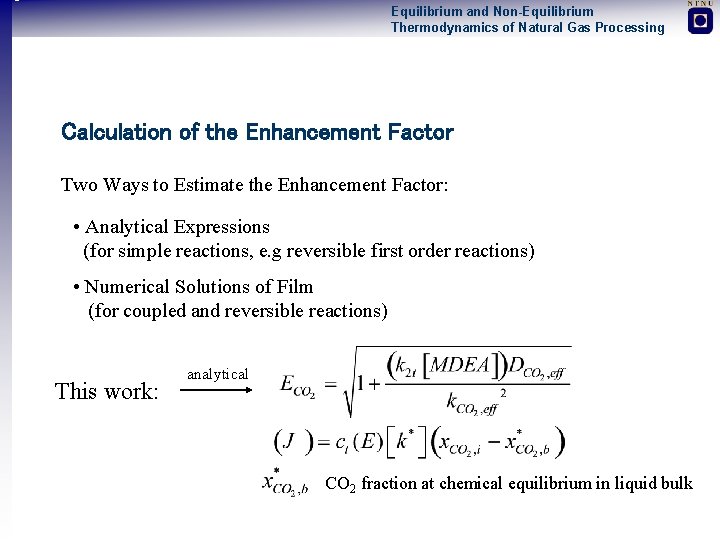
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Calculation of the Enhancement Factor Two Ways to Estimate the Enhancement Factor: • Analytical Expressions (for simple reactions, e. g reversible first order reactions) • Numerical Solutions of Film (for coupled and reversible reactions) This work: analytical CO 2 fraction at chemical equilibrium in liquid bulk
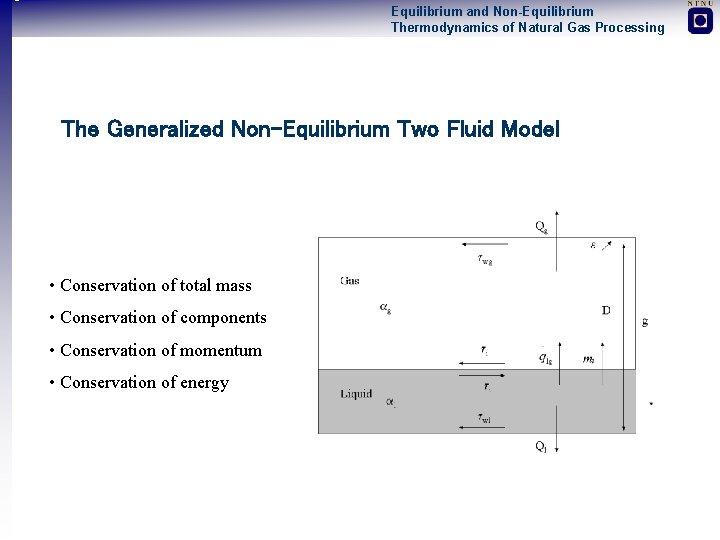
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing The Generalized Non-Equilibrium Two Fluid Model • Conservation of total mass • Conservation of components • Conservation of momentum • Conservation of energy

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Outline 1. Introduction to Natural Gas Processing and Transport 2. Equilibrium and Non-Equilibrium Model Development 3. Presentation of the Simulation Program Developed 4. Modelling and Regression to Experimental Data 5. Experimental Work and Results 6. Conclusions

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Neq. Sim – a General Non-Equilibrium Simulator • General modelling tool for non-equilibrium and equilibrium processes • Based on rigorous thermodynamic models • Fluid mechanics based the one- or two fluid model • Implemented in an object oriented language (Java/Python object oriented design where everything is an object) • Suitable for being used as a modelling tool (general parameter fitting routines implemented) • Validated against experimental data (equilibrium/non-equilibrium)
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Neq. Sim – a General Non-Equilibrium Simulator
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Neq. Sim - Examples of use • Multiphase flash calculation • Construction of phase envelopes • Weak electrolyte calculations • Process plant simulation
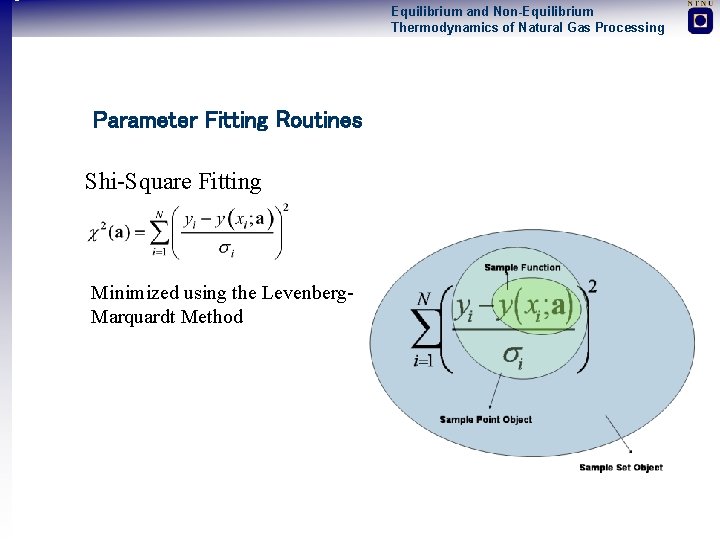
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Parameter Fitting Routines Shi-Square Fitting Minimized using the Levenberg- Marquardt Method

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Outline 1. Introduction to Natural Gas Processing and Transport 2. Equilibrium and Non-Equilibrium Model Development 3. Presentation of the Simulation Program Developed 4. Modelling and Regression to Experimental Data 5. Experimental Work and Results 6. Conclusions
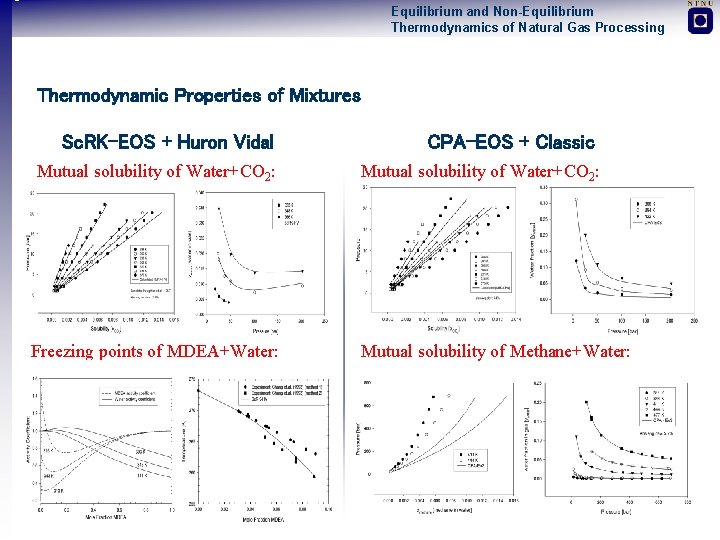
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Thermodynamic Properties of Mixtures Sc. RK-EOS + Huron Vidal CPA-EOS + Classic Mutual solubility of Water+CO 2: Freezing points of MDEA+Water: Mutual solubility of Methane+Water:
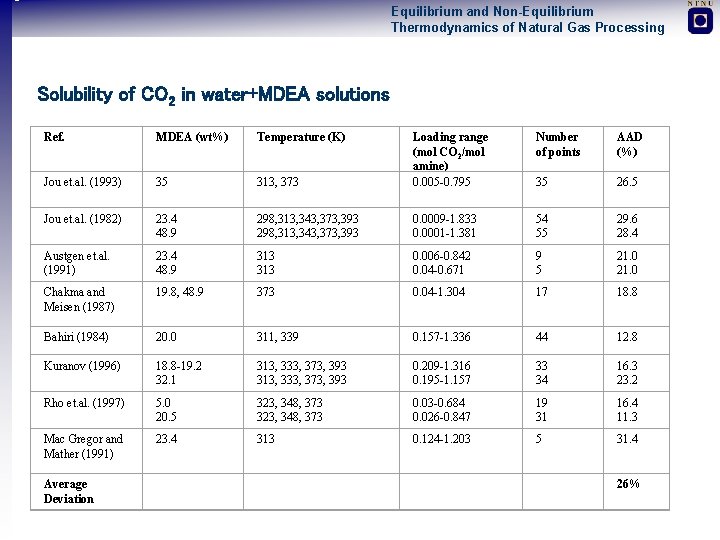
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Solubility of CO 2 in water+MDEA solutions Ref. MDEA (wt%) Temperature (K) Electrolyte Sc. RK-Eo. S Jou et. al. (1993) 35 20. 5 wt % MDEA : 313, 373 Loading range (mol CO 2/mol amine) 0. 005 -0. 795 Number of points Jou et. al. (1982) 23. 4 48. 9 298, 313, 343, 373, 393 0. 0009 -1. 833 0. 0001 -1. 381 54 55 29. 6 28. 4 Austgen et. al. (1991) 23. 4 48. 9 313 0. 006 -0. 842 0. 04 -0. 671 9 5 21. 0 Chakma and Meisen (1987) 19. 8, 48. 9 373 0. 04 -1. 304 17 18. 8 Bahiri (1984) 20. 0 311, 339 0. 157 -1. 336 44 12. 8 Kuranov (1996) 18. 8 -19. 2 32. 1 313, 333, 373, 393 0. 209 -1. 316 0. 195 -1. 157 33 34 16. 3 23. 2 Rho et. al. (1997) 5. 0 20. 5 323, 348, 373 0. 03 -0. 684 0. 026 -0. 847 19 31 16. 4 11. 3 Mac Gregor and Mather (1991) 23. 4 313 0. 124 -1. 203 5 31. 4 Average Deviation 26% 35 50 wt% MDEA AAD (%) 26. 5
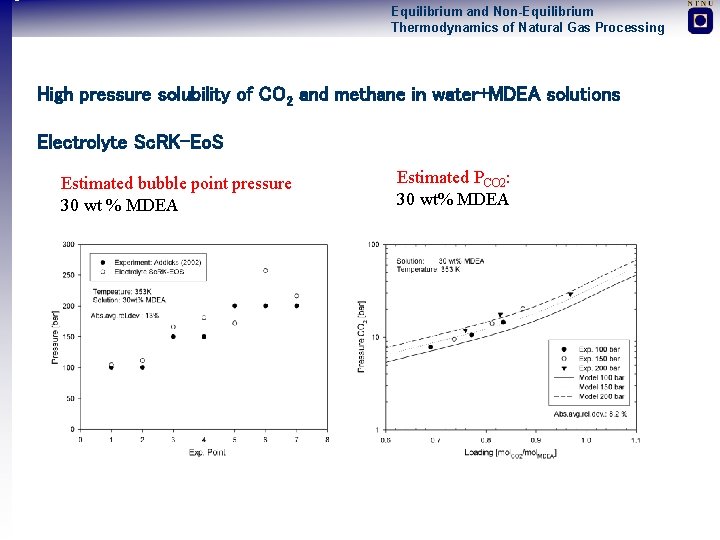
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing High pressure solubility of CO 2 and methane in water+MDEA solutions Electrolyte Sc. RK-Eo. S Estimated bubble point pressure 30 wt % MDEA Estimated PCO 2: 30 wt% MDEA
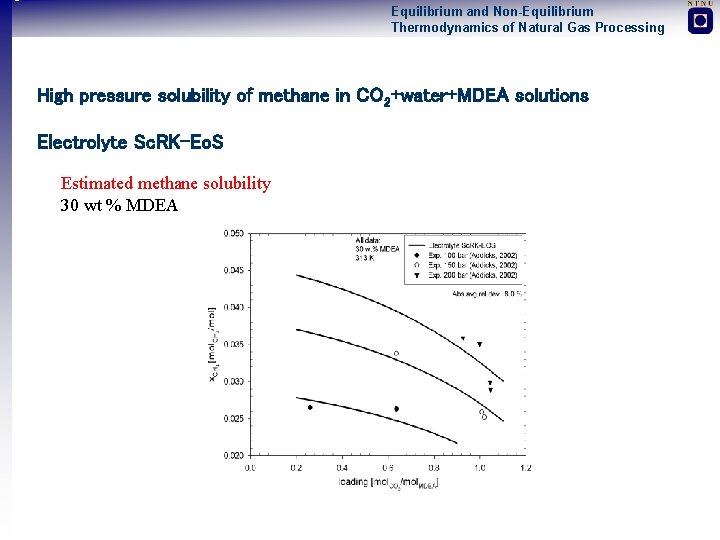
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing High pressure solubility of methane in CO 2+water+MDEA solutions Electrolyte Sc. RK-Eo. S Estimated methane solubility 30 wt % MDEA
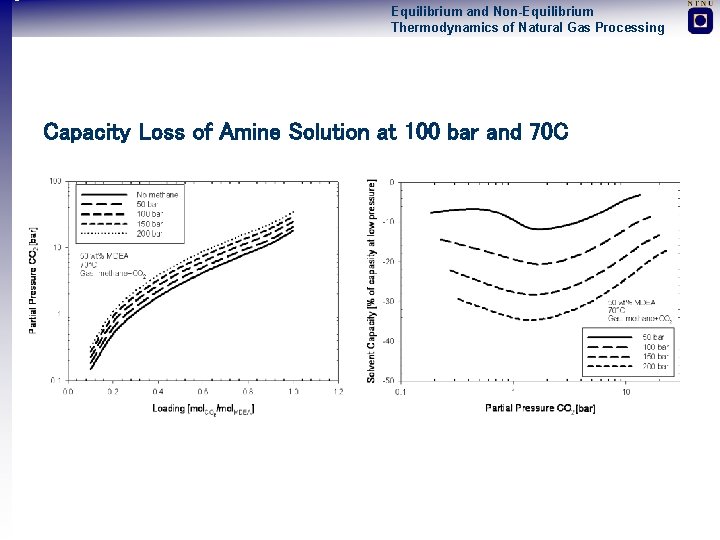
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Capacity Loss of Amine Solution at 100 bar and 70 C

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Outline 1. Introduction to Natural Gas Processing and Transport 2. Equilibrium and Non-Equilibrium Model Development 3. Presentation of the Simulation Program Developed 4. Modelling and Regression to Experimental Data 5. Experimental Work and Results 6. Conclusions
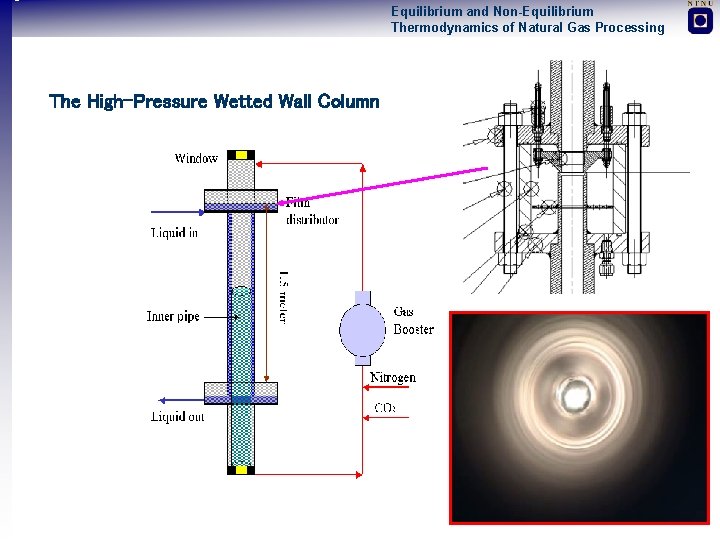
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing The High-Pressure Wetted Wall Column
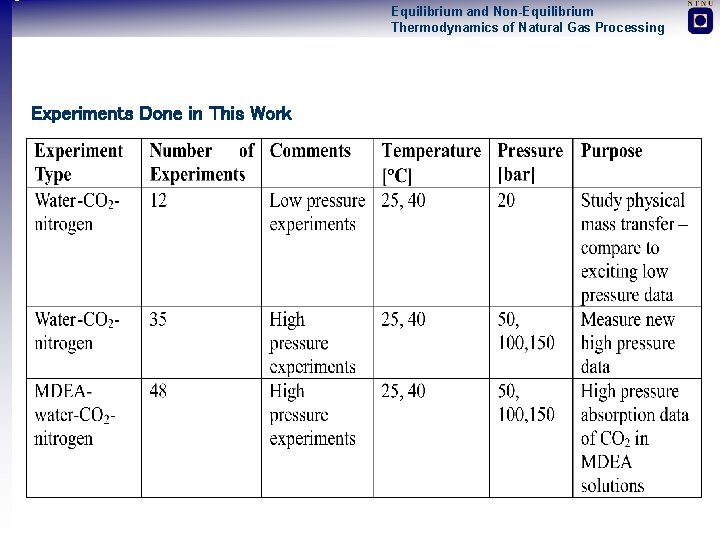
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Experiments Done in This Work
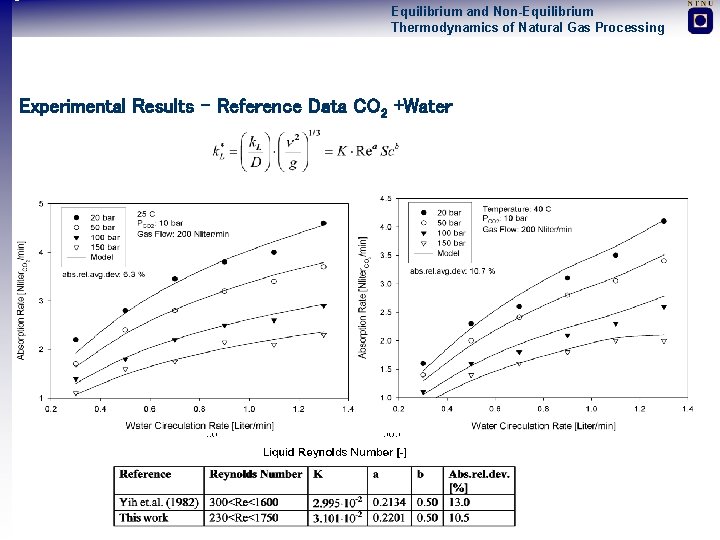
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Experimental Results – Reference Data CO 2 +Water
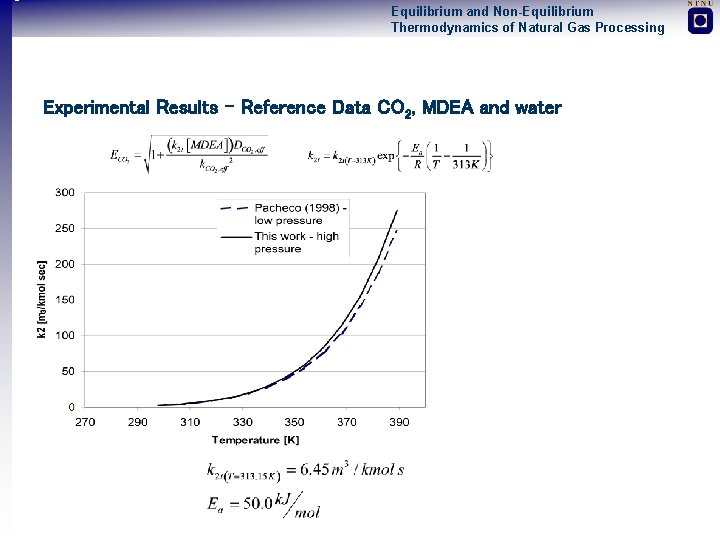
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Experimental Results – Reference Data CO 2, MDEA and water

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Conclusions

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Conclusions
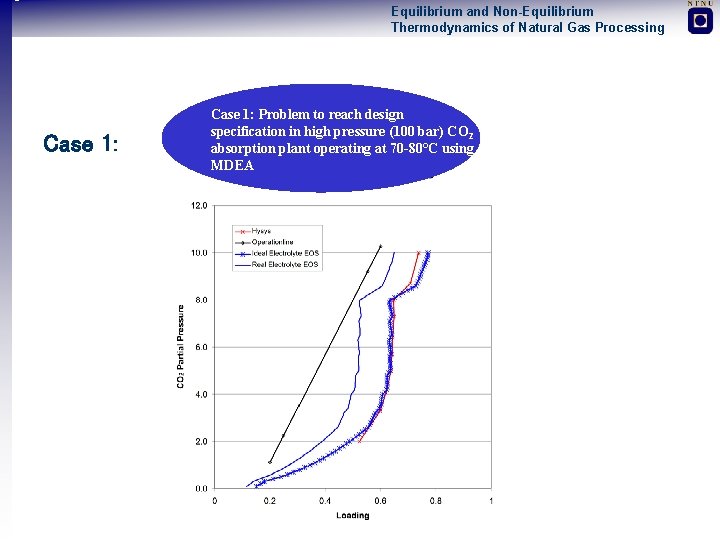
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Case 1: Problem to reach design specification in. Case high 2: pressure (100 bar) CO 2 Water Condensation of Liquid absorption plant operating at Gas 70 -80°C usingoperating in Sub Sea Dry Pipeline MDEA between 100 -200 bar
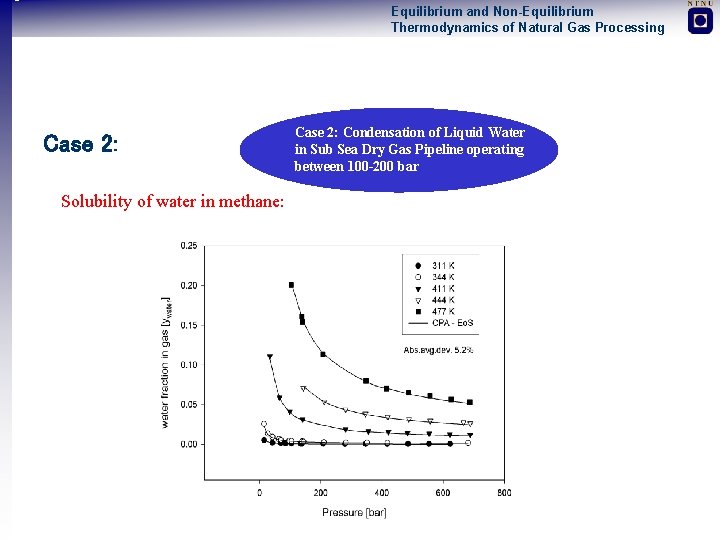
Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Case 2: Solubility of water in methane: Case 2: Condensation of Liquid Water in Sub Sea Dry Gas Pipeline operating between 100 -200 bar

Equilibrium and Non-Equilibrium Thermodynamics of Natural Gas Processing Thanks • Institute for Energy- and Process Technology • Statoil • Norwegian Research Council
- Slides: 49