Equations must be dimensionally consistent What does this

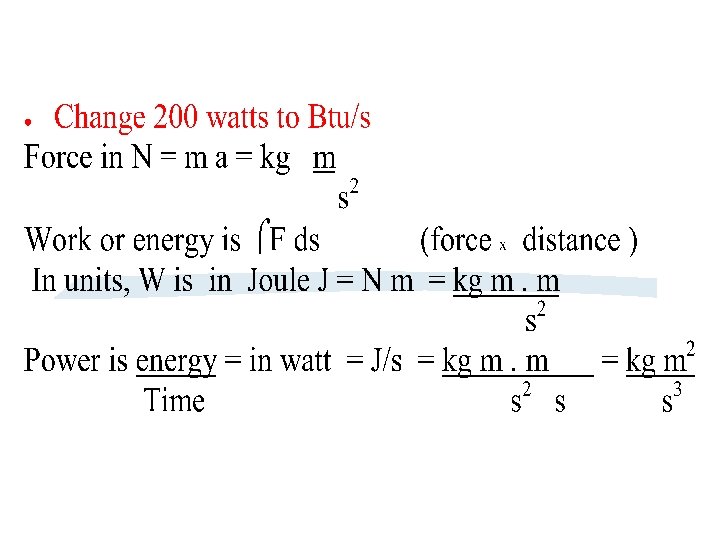

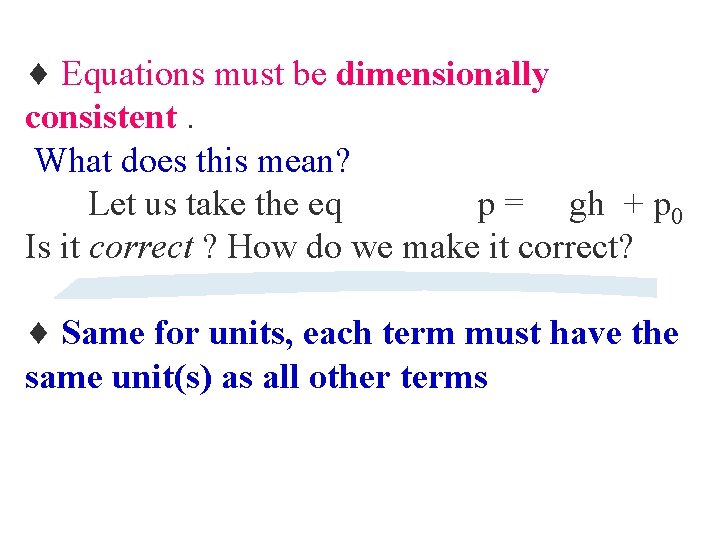
Equations must be dimensionally consistent. What does this mean? Let us take the eq p = gh + p 0 Is it correct ? How do we make it correct? Same for units, each term must have the same unit(s) as all other terms
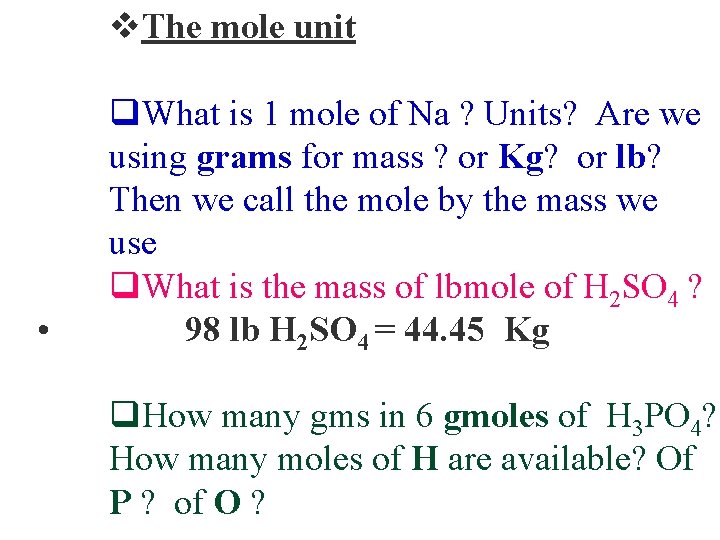
v. The mole unit • q. What is 1 mole of Na ? Units? Are we using grams for mass ? or Kg? or lb? Then we call the mole by the mass we use q. What is the mass of lbmole of H 2 SO 4 ? 98 lb H 2 SO 4 = 44. 45 Kg q. How many gms in 6 gmoles of H 3 PO 4? How many moles of H are available? Of P ? of O ?
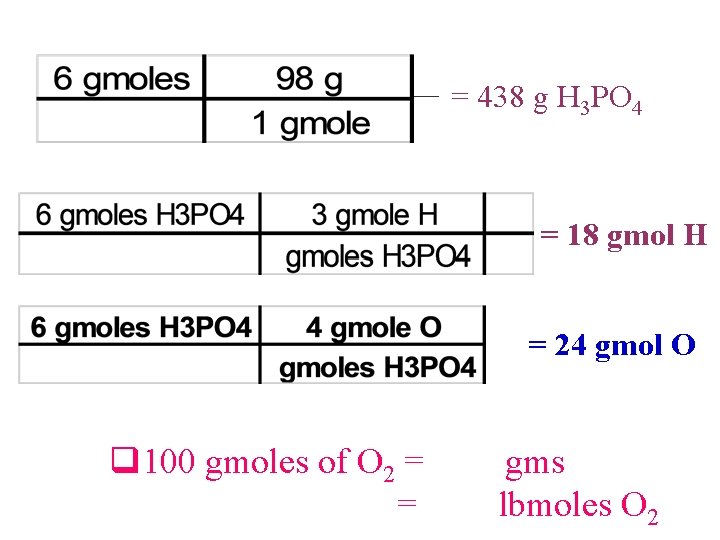
= 438 g H 3 PO 4 = 18 gmol H = 24 gmol O q 100 gmoles of O 2 = = gms lbmoles O 2
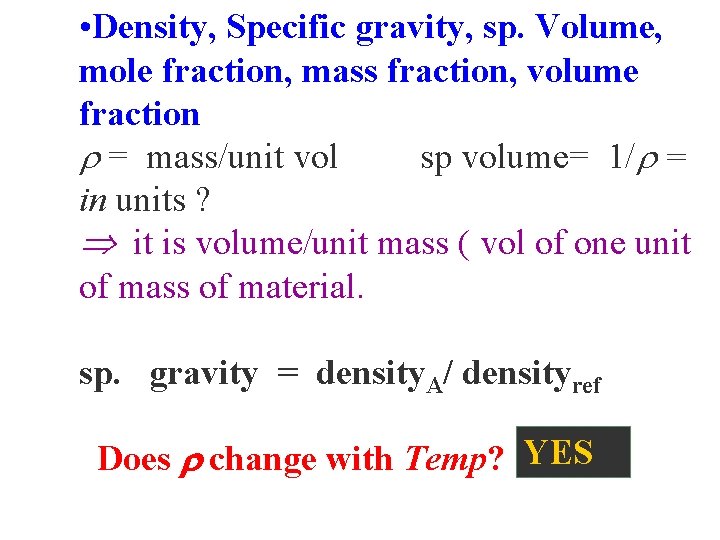
• Density, Specific gravity, sp. Volume, mole fraction, mass fraction, volume fraction = mass/unit vol sp volume= 1/ = in units ? it is volume/unit mass ( vol of one unit of mass of material. sp. gravity = density. A/ densityref Does change with Temp? YES

We need to specify the temp used for A and for the ref. Example sp. gr = 0. 73 20 o 4 o What does this mean ?
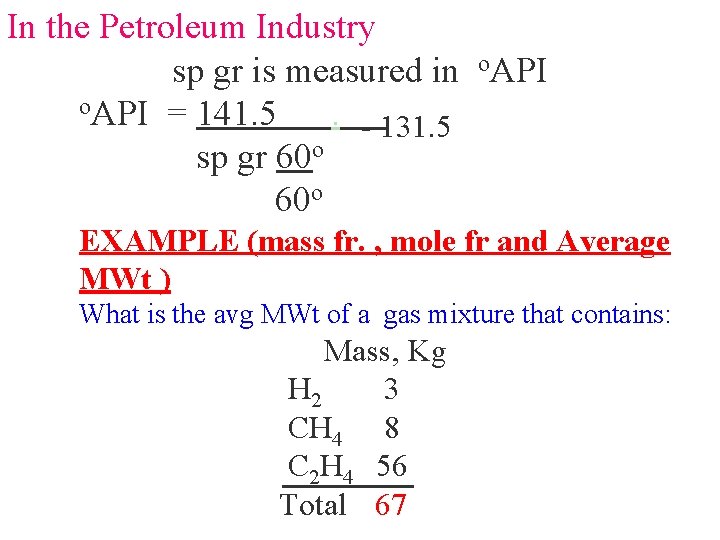
In the Petroleum Industry sp gr is measured in o. API = 141. 5. - 131. 5 sp gr 60 o EXAMPLE (mass fr. , mole fr and Average MWt ) What is the avg MWt of a gas mixture that contains: Mass, Kg H 2 3 CH 4 8 C 2 H 4 56 Total 67
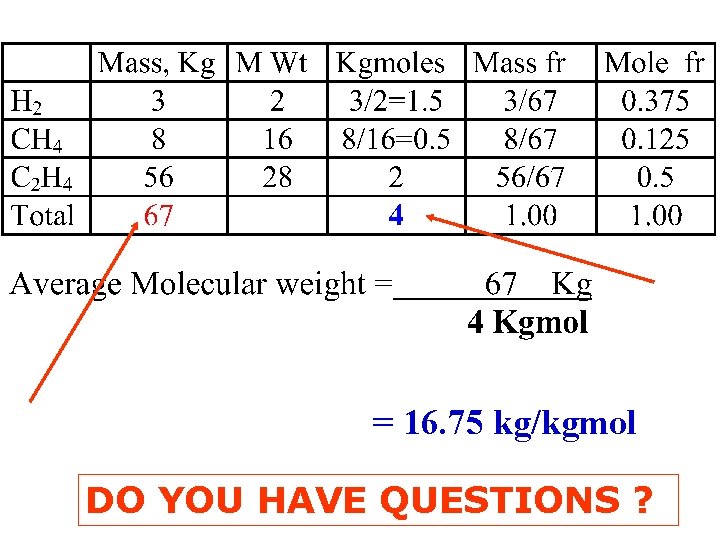
= 16. 75 kg/kgmol DO YOU HAVE QUESTIONS ?
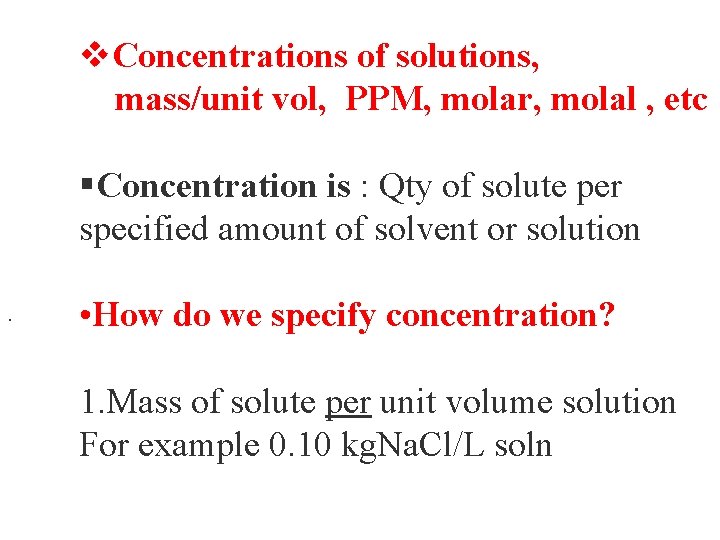
v. Concentrations of solutions, mass/unit vol, PPM, molar, molal , etc §Concentration is : Qty of solute per specified amount of solvent or solution. • How do we specify concentration? 1. Mass of solute per unit volume solution For example 0. 10 kg. Na. Cl/L soln
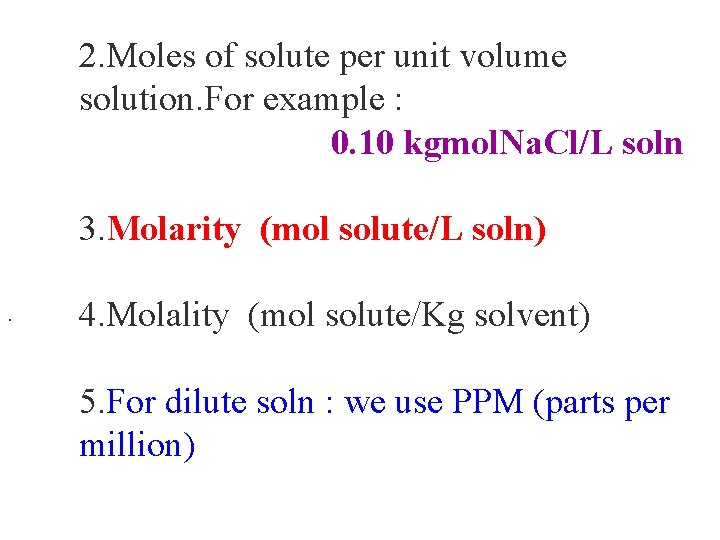
2. Moles of solute per unit volume solution. For example : 0. 10 kgmol. Na. Cl/L soln 3. Molarity (mol solute/L soln). 4. Molality (mol solute/Kg solvent) 5. For dilute soln : we use PPM (parts per million)
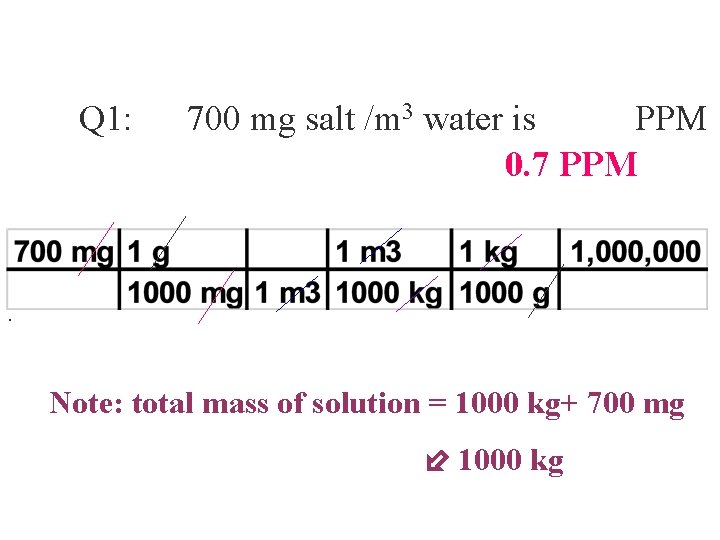
Q 1: 700 mg salt /m 3 water is PPM 0. 7 PPM . Note: total mass of solution = 1000 kg+ 700 mg 1000 kg
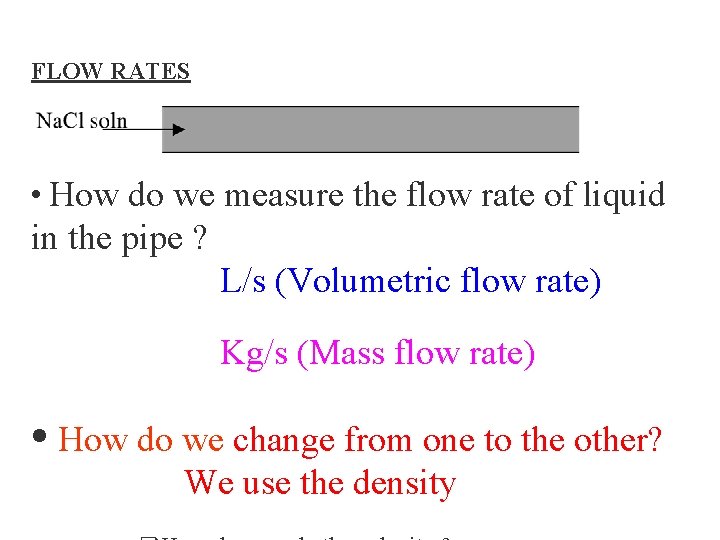
FLOW RATES • How do we measure the flow rate of liquid in the pipe ? L/s (Volumetric flow rate) Kg/s (Mass flow rate) • How do we change from one to the other? We use the density

• How do we calc the velocity ? Q =A. v • Example: 40% Na. Cl soln is flowing at 10 L/min in a pipe. If the sp gr of the soln is 1. 4, then §calculate the concentration of Na. Cl in Kg/L §calculate the flow rate of soln in kgmol soln/min
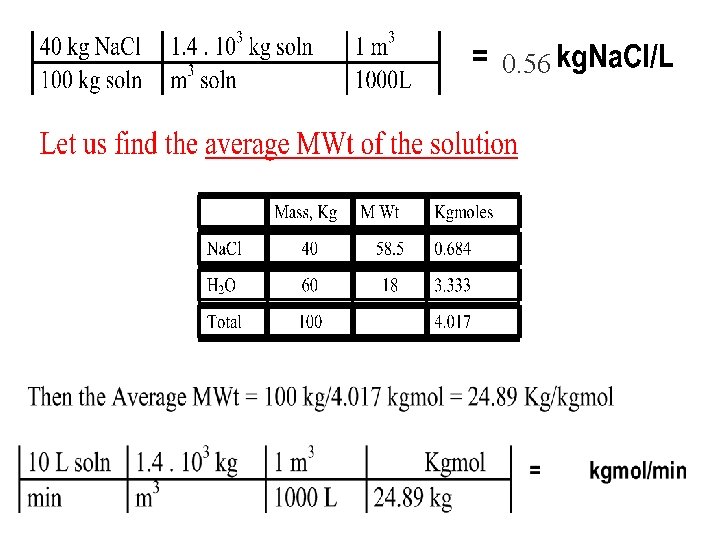
0. 56

Example A gas mixture contains : 40% N 2, 45% O 2 and 15%CH 4. This mixture is hazardous, so to make it safe, it was diluted by adding equal amount of N 2 to it. What is the analysis of the new safe mixture ?
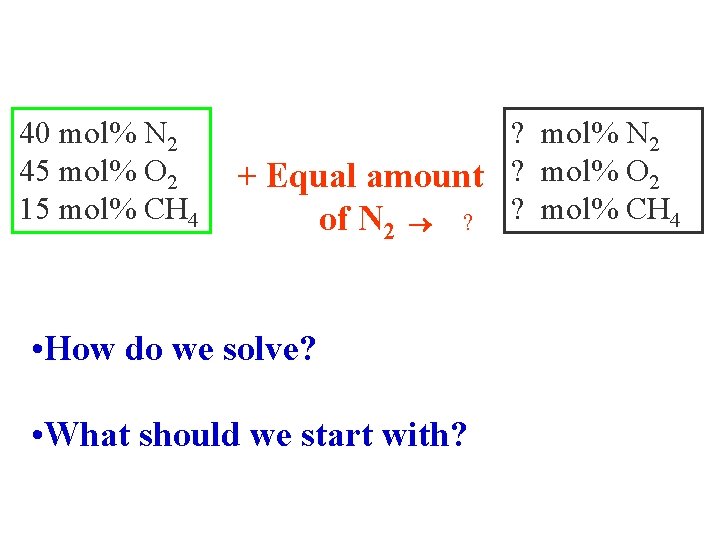
40 mol% N 2 45 mol% O 2 15 mol% CH 4 ? mol% N 2 + Equal amount ? mol% O 2 of N 2 ? ? mol% CH 4 • How do we solve? • What should we start with?

BASIS: 1000 gmoles of starting gas mixture BASIS: 1 lbmole of starting gas mixture BASIS: 50 kgmoles of starting gas mixture
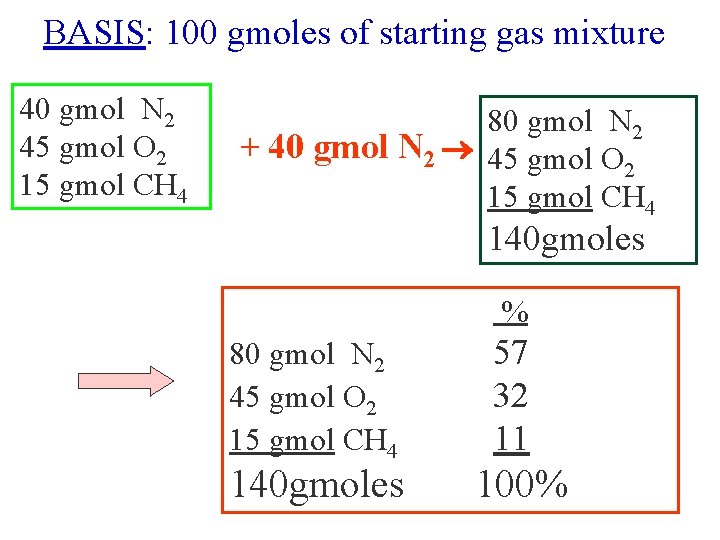
BASIS: 100 gmoles of starting gas mixture 40 gmol N 2 45 gmol O 2 15 gmol CH 4 80 gmol N 2 + 40 gmol N 2 45 gmol O 2 15 gmol CH 4 140 gmoles % 80 gmol N 2 45 gmol O 2 15 gmol CH 4 140 gmoles 57 32 11 100%
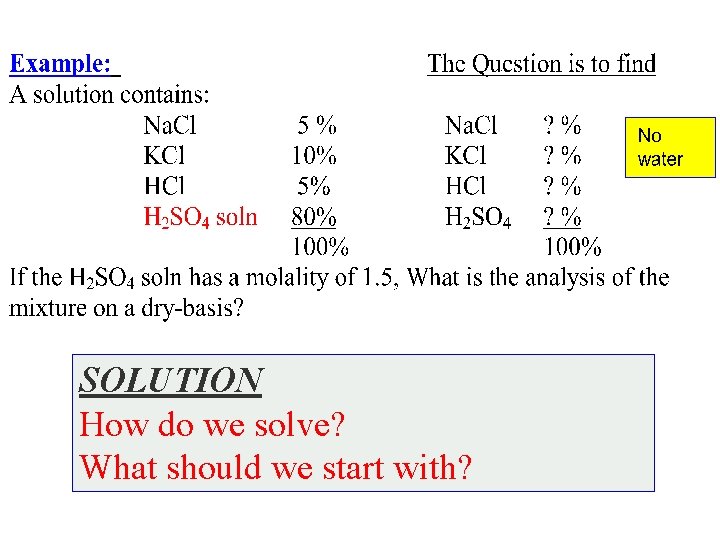
SOLUTION How do we solve? What should we start with?
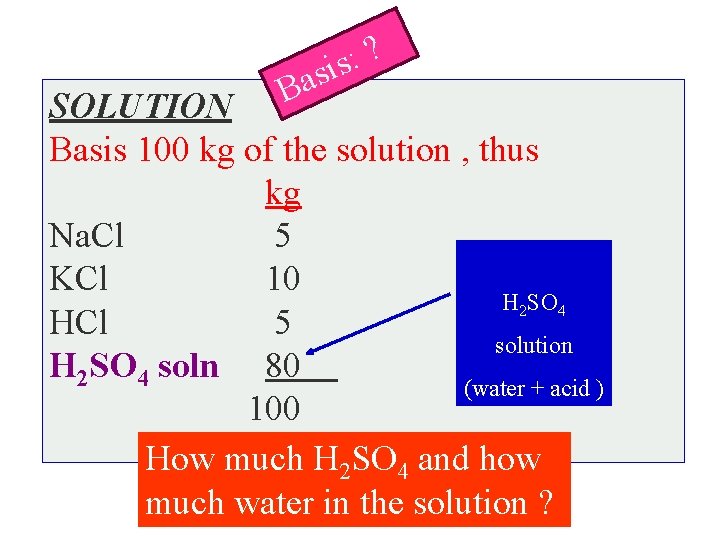
Ba ? : sis SOLUTION Basis 100 kg of the solution , thus kg Na. Cl 5 KCl 10 H 2 SO 4 HCl 5 solution H 2 SO 4 soln 80 (water + acid ) 100 How much H 2 SO 4 and how much water in the solution ?
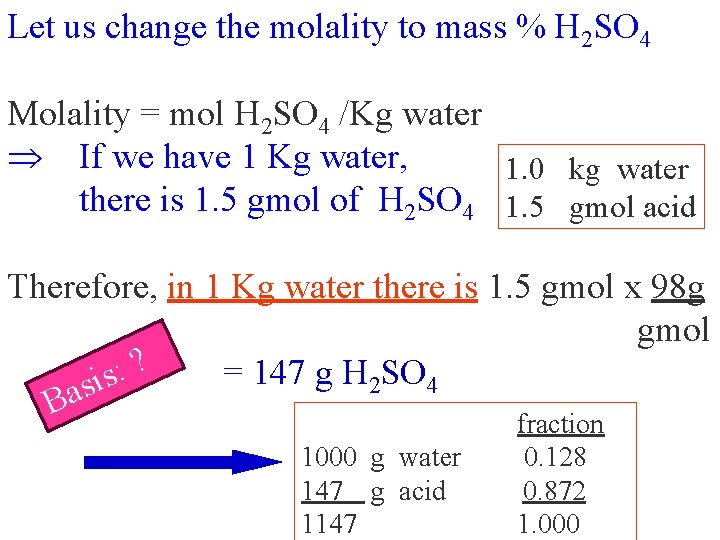
Let us change the molality to mass % H 2 SO 4 Molality = mol H 2 SO 4 /Kg water If we have 1 Kg water, 1. 0 kg water there is 1. 5 gmol of H 2 SO 4 1. 5 gmol acid Therefore, in 1 Kg water there is 1. 5 gmol x 98 g gmol ? : = 147 g H SO s i 2 4 s a B fraction 1000 g water 147 g acid 1147 0. 128 0. 872 1. 000
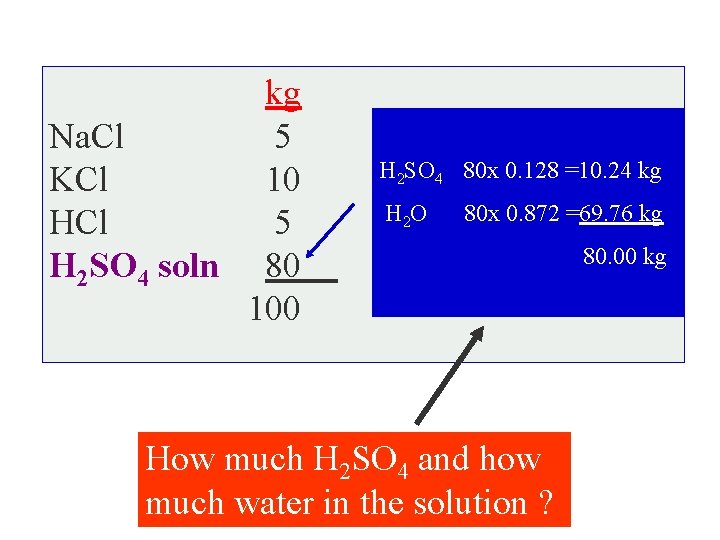
kg Na. Cl 5 KCl 10 HCl 5 H 2 SO 4 soln 80 100 H 2 SO 4 80 x 0. 128 =10. 24 kg H 2 O 80 x 0. 872 =69. 76 kg How much H 2 SO 4 and how much water in the solution ? 80. 00 kg
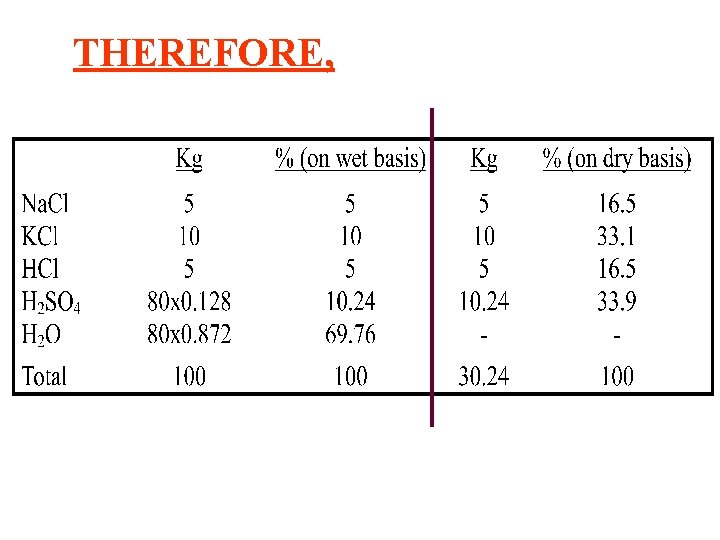
THEREFORE,


- Slides: 27