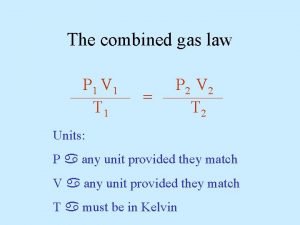
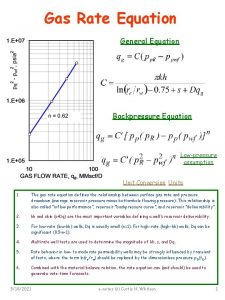
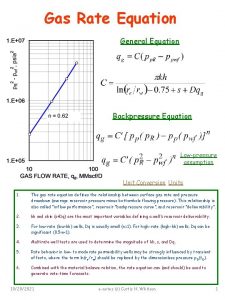
Equation of State Real Gas Relationships Relates PVT







- Slides: 15

Equation of State Real Gas Relationships

• Relates PVT properties of a pure substance/mixture via Theoratical and empirical relations • Source of information: computer databases • Effective Eq. of state: experimental data with good precision


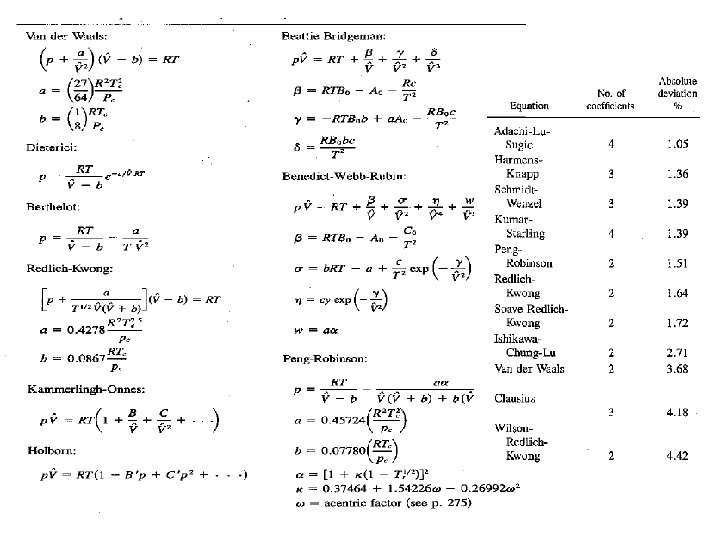
Van-der Waals Equation • • Simple, explains computational problems. Also illustrates Theoretical developments • • Fortan computer Programs for non linear equations One Code: Newton Method, Other Minimization


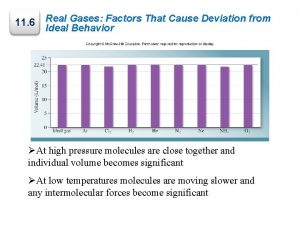
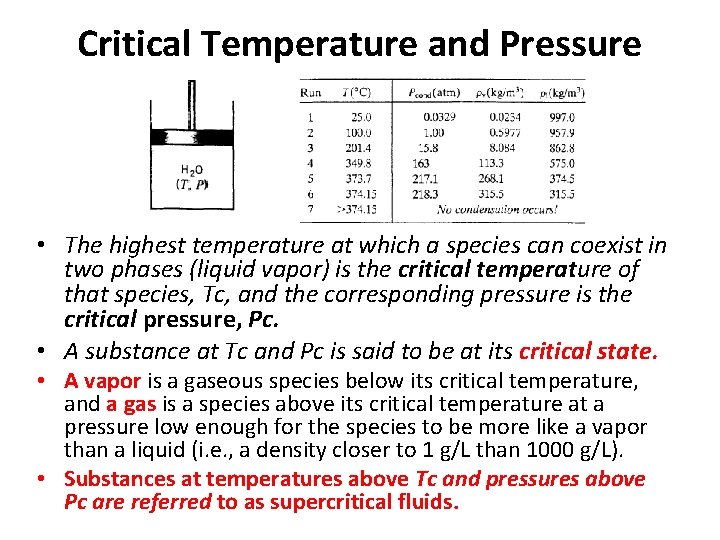
Critical Temperature and Pressure • The highest temperature at which a species can coexist in two phases (liquid vapor) is the critical temperature of that species, Tc, and the corresponding pressure is the critical pressure, Pc. • A substance at Tc and Pc is said to be at its critical state. • A vapor is a gaseous species below its critical temperature, and a gas is a species above its critical temperature at a pressure low enough for the species to be more like a vapor than a liquid (i. e. , a density closer to 1 g/L than 1000 g/L). • Substances at temperatures above Tc and pressures above Pc are referred to as supercritical fluids.

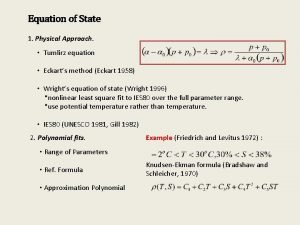
Cubic Equation of State • Fit the experimental data with as few constants in equation as possible • Concise summary of a large of experimental data • Accurate interpolation between experimental data points • Provide a continuous function to facilitate calculation of physical properties involving differentiation and integration. • Point of departure for the treatment of thermodynamic properties of mixtures.

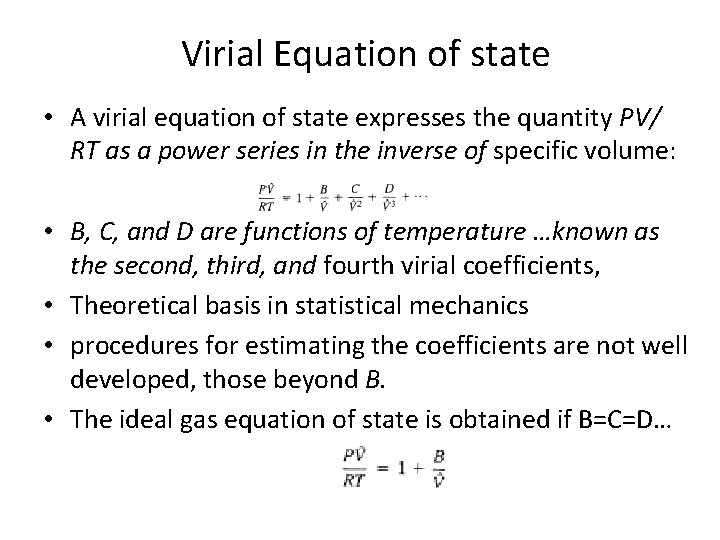
Virial Equation of state • A virial equation of state expresses the quantity PV/ RT as a power series in the inverse of specific volume: • B, C, and D are functions of temperature …known as the second, third, and fourth virial coefficients, • Theoretical basis in statistical mechanics • procedures for estimating the coefficients are not well developed, those beyond B. • The ideal gas equation of state is obtained if B=C=D…

• For polar compounds (asymmetrical compounds with a nonzero dipole moment, such as water). • Can estimate V or P for a given T for a nonpolar species (one with a dipole moment close to zero, such as hydrogen and oxygen and all other molecularly symmetrical compounds). • Solution for P is straightforward. • For V, the equation can be rearranged into a quadratic and solved using the quadratic formula. • One of the two solutions is reasonable and the other is not and should be discarded; • if there is any doubt, estimate V from the ideal gas equation of state and accept the virial equation solution that comes closest to V ideal'

Steps Involved • Look up the critical temperature and pressure (Tc and Pc) for the species of interest • Pitzer acentric factor, w. a parameter that reflects the geometry and polarity of a molecule. • Calculate the reduced temperature, Tr = TITc. • Estimate B using the following equations • Substitute into Equation the values of B and whichever of the variables P and V is known and solve for the other variable

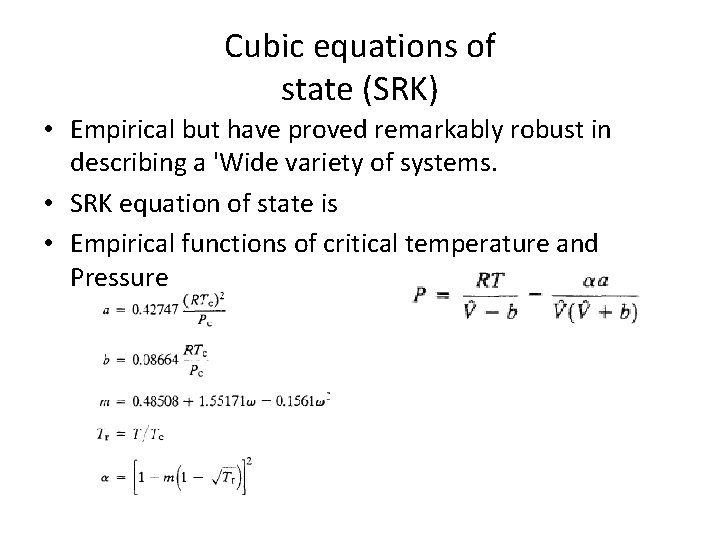
Cubic equations of state (SRK) • Empirical but have proved remarkably robust in describing a 'Wide variety of systems. • SRK equation of state is • Empirical functions of critical temperature and Pressure

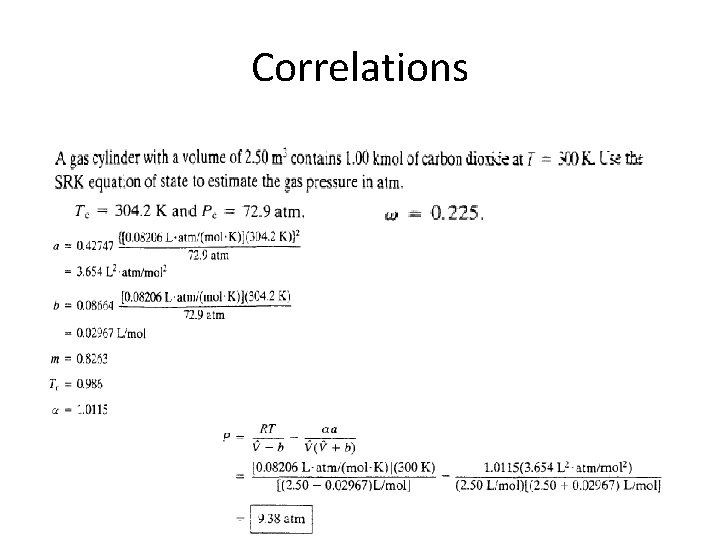
Correlations

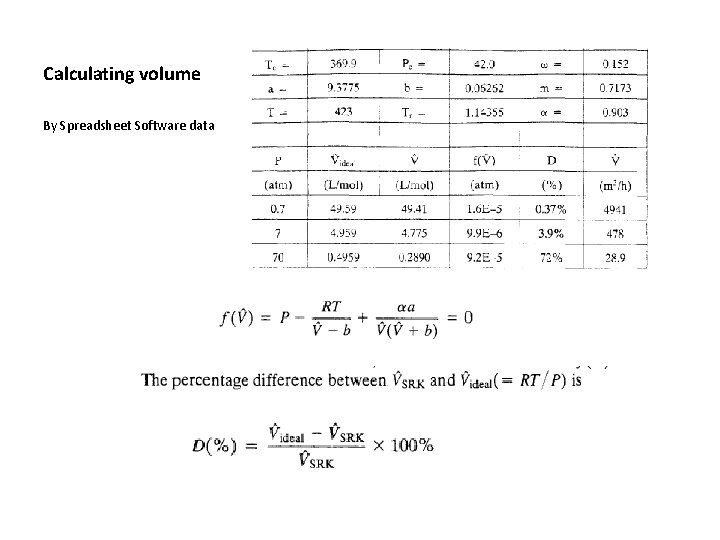
Calculating volume By Spreadsheet Software data


Considerations • SRK equation of state (and every other equation of state) is itself an • approximation. • All equations of state have parameters obtained by fitting empirical expressions to experimental PVT data. • The fit may be excellent in the temperature and pressure ranges where the data were obtained but may be terrible elsewhere. • Always try to ascertain the region of validity of any equation of state intend to be used. • No assurance of the accuracy of the equation for condition far from acceptable region.
 Equation of state of real gas
Equation of state of real gas What gas law relates pressure and temperature
What gas law relates pressure and temperature Chapter 13 gases
Chapter 13 gases Pvt=pvt
Pvt=pvt Inverse variation parent function
Inverse variation parent function Differences between ideal gas and real gas
Differences between ideal gas and real gas Difference between ideal gas and real gas
Difference between ideal gas and real gas Real gas equation
Real gas equation Van der waals real gas equation
Van der waals real gas equation Genesis gas solutions
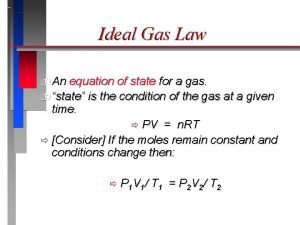
Genesis gas solutions Ideal gas equation of state
Ideal gas equation of state Condition of ideal gas
Condition of ideal gas Green theorem and stokes theorem are same
Green theorem and stokes theorem are same Fick's law of diffusion
Fick's law of diffusion What formula relates moles, mass and mr?
What formula relates moles, mass and mr? Hooke's law relates
Hooke's law relates