EPITHELIALIZATION Epithelial healing or epithelialization which begins a
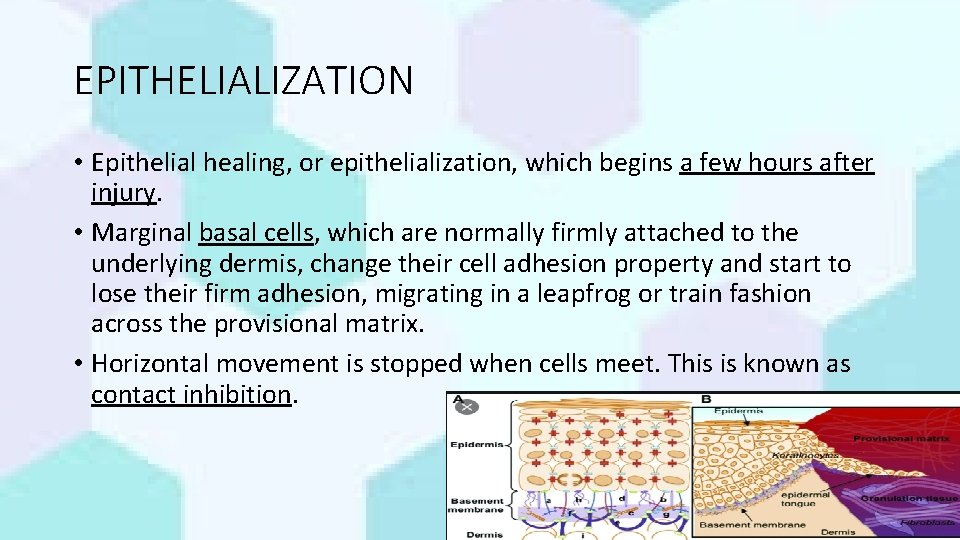
EPITHELIALIZATION • Epithelial healing, or epithelialization, which begins a few hours after injury. • Marginal basal cells, which are normally firmly attached to the underlying dermis, change their cell adhesion property and start to lose their firm adhesion, migrating in a leapfrog or train fashion across the provisional matrix. • Horizontal movement is stopped when cells meet. This is known as contact inhibition. Dr. Kanwal 1
WOUND CONTRACTION • The final feature of the proliferation phase, normally starts 5 days after injury. • Wound contraction appears to be a dynamic process in which cells organize their surrounding connective tissue matrix, acting to reduce the healing time by reducing the amount of ECM that needs to be produced. • The contractile activity of fibroblasts and myofibroblasts provides the force for this contraction. • These cells may use integrins and other adhesion mechanisms to bind to the collagen network and alter its motility, bringing the fibrils and, subsequently, the wound edges closer. Dr. Kanwal 2
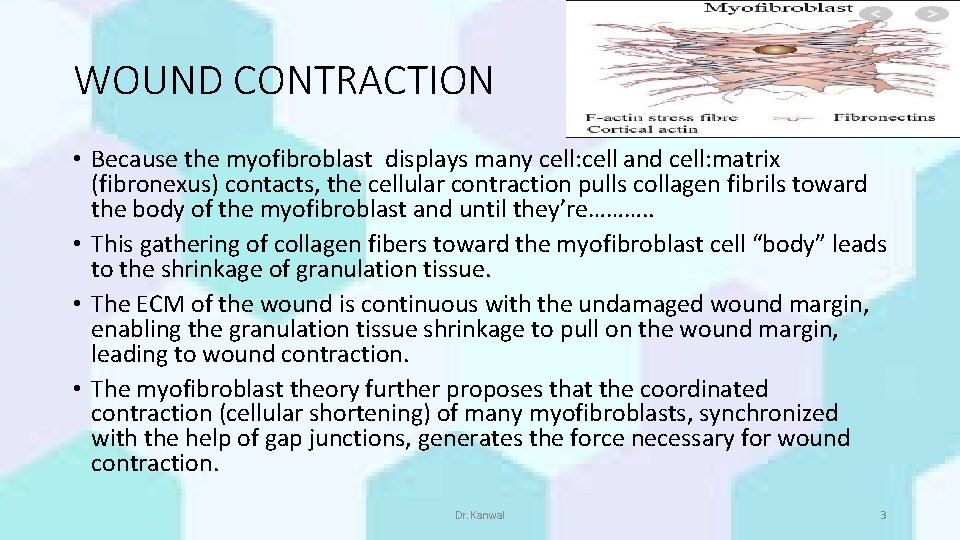
WOUND CONTRACTION • Because the myofibroblast displays many cell: cell and cell: matrix (fibronexus) contacts, the cellular contraction pulls collagen fibrils toward the body of the myofibroblast and until they’re………. . • This gathering of collagen fibers toward the myofibroblast cell “body” leads to the shrinkage of granulation tissue. • The ECM of the wound is continuous with the undamaged wound margin, enabling the granulation tissue shrinkage to pull on the wound margin, leading to wound contraction. • The myofibroblast theory further proposes that the coordinated contraction (cellular shortening) of many myofibroblasts, synchronized with the help of gap junctions, generates the force necessary for wound contraction. Dr. Kanwal 3

WOUND CONTRACTION • The traction theory proposes that fibroblasts bring about a closer approximation of matrix fibrils by exerting “traction forces” (analogous to the traction of wheels on tarmac) on extracellular matrix fibers to which they’re attached. • This theory proposes that fibroblasts neither shorten in length nor act in a coordinated multicellular manner (as proposed by the myofibroblast theory); rather, a composite force, made up of traction forces of many individual fibroblasts, is responsible for matrix contraction. • Such traction forces act as shearing forces tangential to the cell surface generated during cell elongation and spreading. • According to the traction theory, the composite effect of many fibroblasts gathering collagen fibrils within the wound is thought to bring about wound contraction. Dr. Kanwal 4
Maturation phase • The maturation phase normally starts 7 days after injury and may last for 1 year or more. • The initial component in the deposited ECM is fibronectin, which forms a provisional fiber network. • Other components include hyaluronic acid and proteoglycans. • The network has two main roles: as a substratum for the migration and growth of cells and as a template for subsequent collagen deposition. • Collagen deposition becomes the predominant constituent of the matrix and soon forms fibrillar bundles and provides stiffness and tensile strength to the wound. Dr. Kanwal 5
Maturation phase • Within 3 weeks of injury, the tensile strength is restored to approximately 20% of normal, uninjured skin. As healing continues, the skin gradually reaches a maximum of 70% to 80% tensile strength. • Different organs regain tensile strengths to differing degrees. • The remodeling process involves the balance between the synthesis and degradation of collagen. • A range of collagenases regulates the latter. • This process is also characterized by a gradual reduction in cellularity and vascularity. • Differentiation of fibroblasts into myofibroblasts with resultant apoptosis (programmed cell death) are also features of tissue remodeling Dr. Kanwal 6
• The scar is the final product of wound healing and is a relatively avascular and acellular mass of collagen that serves to restore tissue continuity and some degree of tensile strength and function. • However, the strength of the scar remains less than that of normal tissue, even many years following injury, and it’s never fully restored. Dr. Kanwal 7
Dr. Kanwal 8

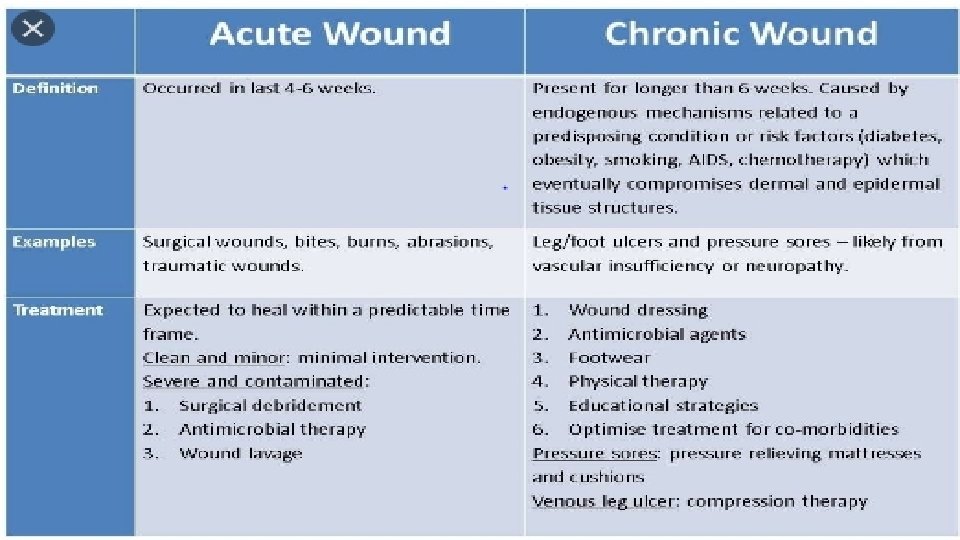
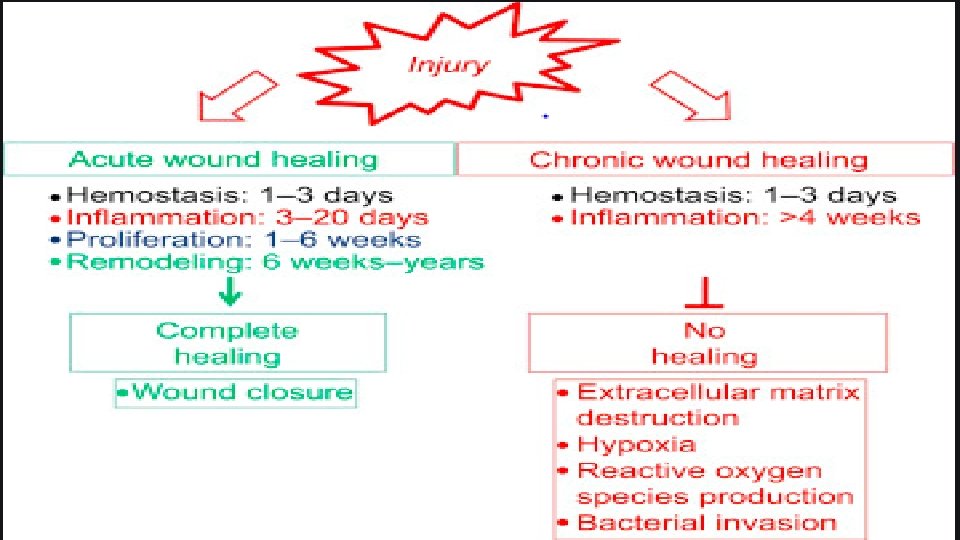
ACUTE vs. CHRONIC WOUND HEALING • Molecular and cellular abnormalities in chronic wounds • Chronicity implies a prolonged or lengthy healing process, whereas acute implies uncomplicated, orderly or organized, or rapid healing. • An acute wound is defined as : • “a disruption in the integrity of the skin and underlying tissues that progresses through the healing process in a timely and uncomplicated manner. ” • Typically, surgical and traumatic wounds, which heal by primary intention, are classified as acute • chronic wound is defined as: • “one that deviates from expected sequence of repair in terms of time, appearance, and response to aggressive and appropriate treatment. ” • Chronic wounds are wounds that “fail to progress through a normal, orderly, and timely sequence of repair or wounds that pass through the repair process without restoring anatomic and functional results. ” • Such wounds usually heal by secondary intention and are associated with pathology; for example, diabetes, ischemic disease, pressure damage, and Dr. Kanwal 9 inflammatory diseases.
ACUTE vs. CHRONIC WOUND HEALING • Prolonged inflammation is the most significant factor in delayed healing. • The prolonged inflammatory phase is due to the presence of inflammatory leukocytes, typically neutrophils and their production of proinflammatory cytokines that perpetuate inflammation. • It is also argued that the release of tissue- damaging proteinases, which degrade newly formed tissue, delay or prevent normal wound healing processes. • In addition to prolonged inflammation, several other factors that may induce chronicity, including recurrent physical trauma, ischemic reperfusion injury, subclinical bacterial contamination, and foreign bodies. • Because chronic wounds are typically characterized by full-thickness tissue loss, • Re-epithelialization is prolonged due to the loss of appendages. • Normally, epithelial cells require the smooth, moist surface of the basement membrane to move across the wound. • In chronic wounds, epithelial cells latch onto and pull themselves across the scaffolding of macromolecules of the provisional matrix, such as Dr. Kanwal 10 laminin and fibronectin.
Dr. Kanwal 11
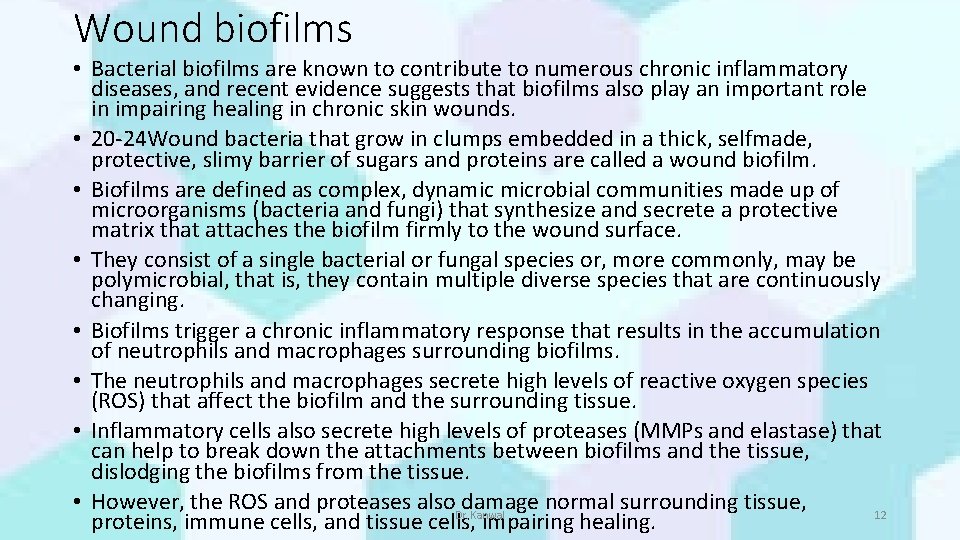
Wound biofilms • Bacterial biofilms are known to contribute to numerous chronic inflammatory diseases, and recent evidence suggests that biofilms also play an important role in impairing healing in chronic skin wounds. • 20 -24 Wound bacteria that grow in clumps embedded in a thick, selfmade, protective, slimy barrier of sugars and proteins are called a wound biofilm. • Biofilms are defined as complex, dynamic microbial communities made up of microorganisms (bacteria and fungi) that synthesize and secrete a protective matrix that attaches the biofilm firmly to the wound surface. • They consist of a single bacterial or fungal species or, more commonly, may be polymicrobial, that is, they contain multiple diverse species that are continuously changing. • Biofilms trigger a chronic inflammatory response that results in the accumulation of neutrophils and macrophages surrounding biofilms. • The neutrophils and macrophages secrete high levels of reactive oxygen species (ROS) that affect the biofilm and the surrounding tissue. • Inflammatory cells also secrete high levels of proteases (MMPs and elastase) that can help to break down the attachments between biofilms and the tissue, dislodging the biofilms from the tissue. • However, the ROS and proteases also. Dr. Kanwal damage normal surrounding tissue, 12 proteins, immune cells, and tissue cells, impairing healing.
Dr. Kanwal 13

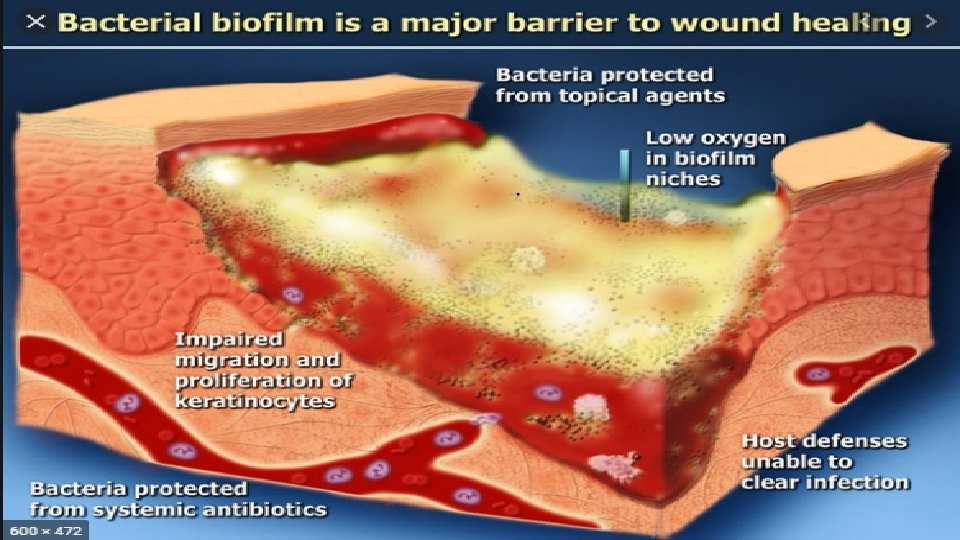
PREDISPOSING FACTORS FOR DEVELOPMENT OF WOUND BIOFILMS • In vulnerable tissue, biofilms arise from planktonic bacteria attaching and forming a protective community before they are killed by the patient’s immune system, by antibiotics, or by debridement. • Thus, general conditions that impair the immune system or reduce the effectiveness of antibiotic drugs favor the development of biofilms in wounds. • These conditions include ischemia or necrosis of tissue; poor patient nutrition; co- morbidities that impair immune function, such as HIV, diabetes, major trauma, radiation treatment; or treatment with immunesuppressing drugs. Dr. Kanwal 14
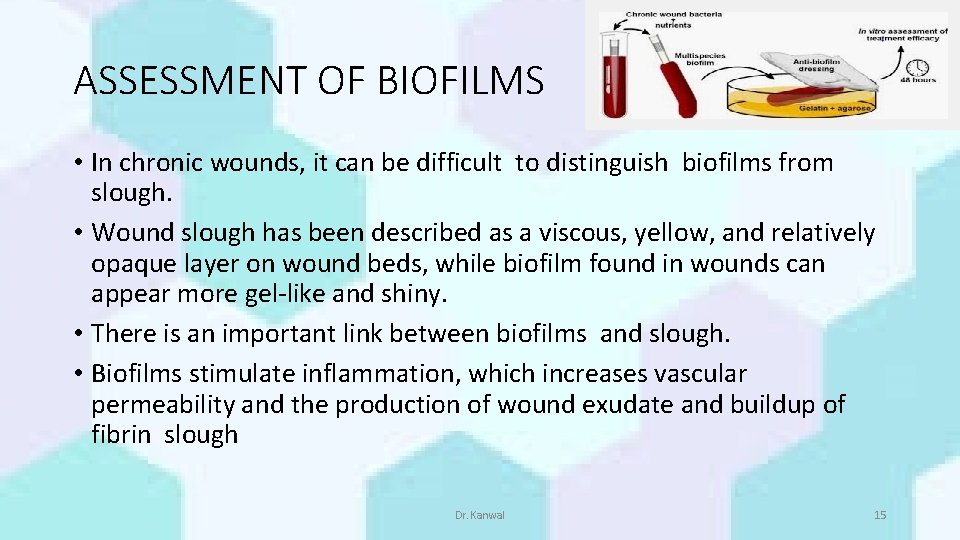
ASSESSMENT OF BIOFILMS • In chronic wounds, it can be difficult to distinguish biofilms from slough. • Wound slough has been described as a viscous, yellow, and relatively opaque layer on wound beds, while biofilm found in wounds can appear more gel-like and shiny. • There is an important link between biofilms and slough. • Biofilms stimulate inflammation, which increases vascular permeability and the production of wound exudate and buildup of fibrin slough Dr. Kanwal 15
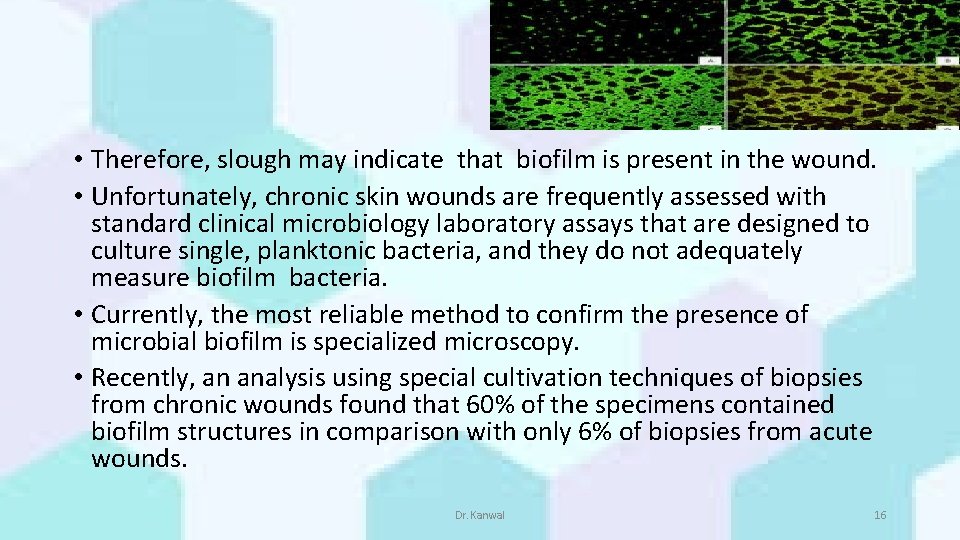
• Therefore, slough may indicate that biofilm is present in the wound. • Unfortunately, chronic skin wounds are frequently assessed with standard clinical microbiology laboratory assays that are designed to culture single, planktonic bacteria, and they do not adequately measure biofilm bacteria. • Currently, the most reliable method to confirm the presence of microbial biofilm is specialized microscopy. • Recently, an analysis using special cultivation techniques of biopsies from chronic wounds found that 60% of the specimens contained biofilm structures in comparison with only 6% of biopsies from acute wounds. Dr. Kanwal 16
MANAGEMENT OF BIOFILMS • Antibiotics and antiseptics kill single bacteria very easily, but the biofilm barrier blocks most antibiotics and antiseptics from reaching the bacteria, particularly in the center of the wound matrix. • Wound biofilms are resistant to antibodies, antibiotics, disinfectants, and phagocytic inflammatory cells. • Wound biofilms can be effectively treated by a combination of debridement and/or cleansing to remove the biofilms, followed by application of dressings that block new bacteria from reaching the wound and killing bacteria left in the wound bed. • These treatments can heal wounds, but patients must comply with the treatment plan because biofilms can re-form within a day and the wound will not heal. Dr. Kanwal 17
Dr. Kanwal 18
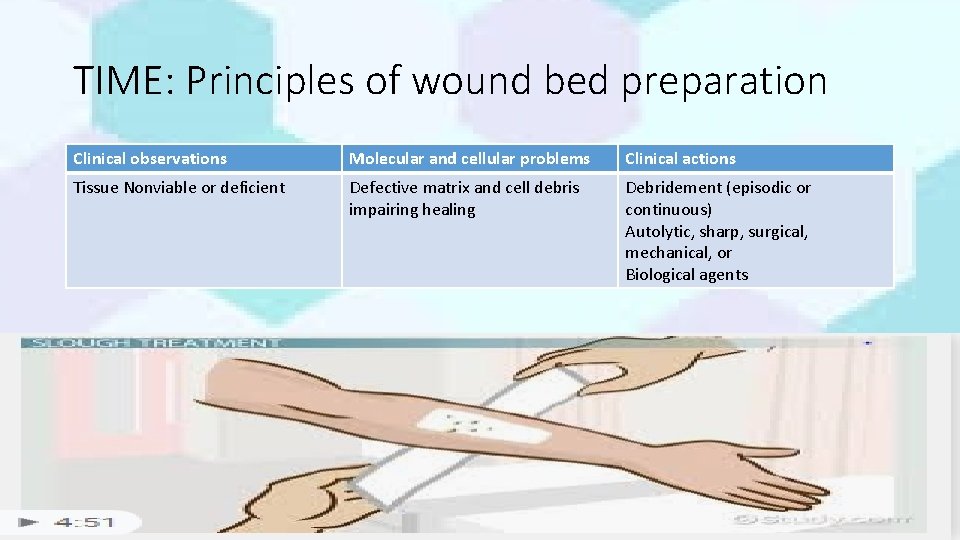
TIME: Principles of wound bed preparation Clinical observations Molecular and cellular problems Clinical actions Tissue Nonviable or deficient Defective matrix and cell debris impairing healing Debridement (episodic or continuous) Autolytic, sharp, surgical, mechanical, or Biological agents Dr. Kanwal 19
- Slides: 19