EPIDEMIOLOGY The study of Frequency l Distribution l

EPIDEMIOLOGY The study of : Frequency l Distribution l Determinants l of health-related states or events

Overview of Epidemiologic Study Designs

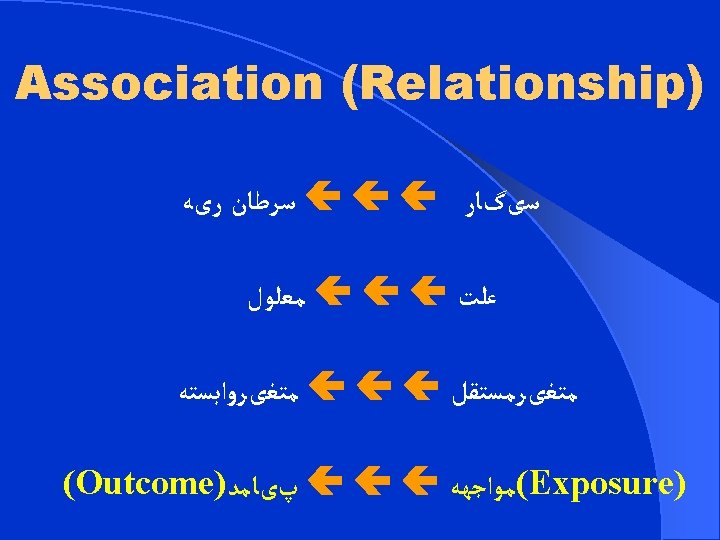
( ﺍﻱ )ﺗﺠﺮﺑﻲ ﻣﺪﺍﺧﻠﻪ ﻳﺎ ﺍﻱ ﻣﺸﺎﻫﺪﻩ (EXPRERIMENTAL) INTERVENTIONAL OBSERVATIONAL ﺗﺤﻠﻴﻠﻲ ﻳﺎ ﺗﻮﺻﻴﻔﻲ ANALYTICAL DESCRIPTIVE ﻃﻮﻟﻲ ﻳﺎ ﻣﻘﻄﻌﻲ LONGITUDINAL CROSS-SECTIONAL


The Main Types of Epidemiologic Study Designs • Cross - Sectional • Case - Control • Cohort • Clinical Trial

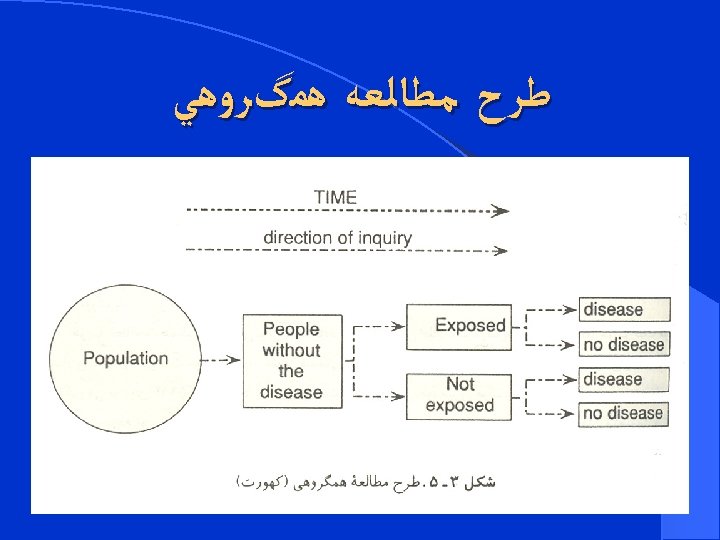
COHORT STUDY

Cohort study l. Cohort study is a design (type) of research method used to investigate the causes of disease and to establish links between risk factors and health outcomes.

Definition l Syn: prospective study, incidence study, follow-up study , longitudinal study. l A cohort study is an observational , longitudinaland usully analytical study. l The goal of a cohort study is to measure and often to compare the incidence of disease in one or more study cohorts.

WHAT IS A COHORT ? Ancient Roman military unit, A band. Persons banded together. A cohort is a group of people who share a common characteristic or experience within a defined period (e. g. , age, job).
Cohort Characteristics l Large sample l Time consuming l Expensive l Highest level of evidence(causality) l Multiple exposures and multiple outcomes l Analysis

TYPES OF COHORT STUDIES l Prospective Cohort (Concurrent) l Retrospective Cohort (Historical) l Ambidirectional Cohort
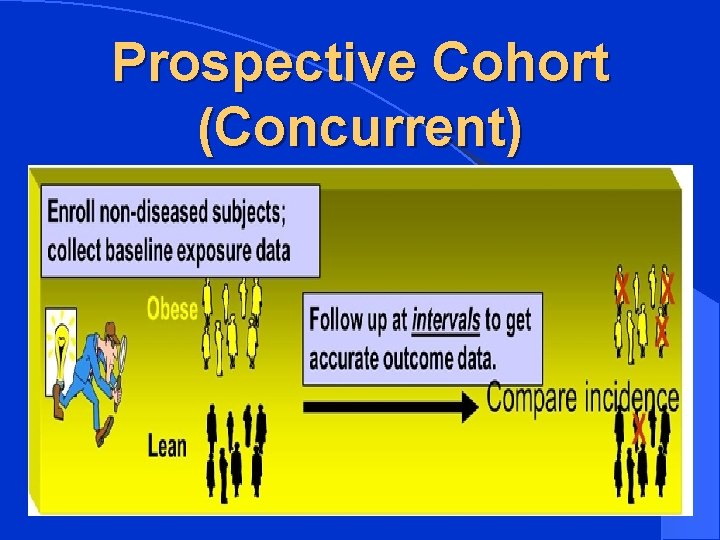
Prospective Cohort (Concurrent)
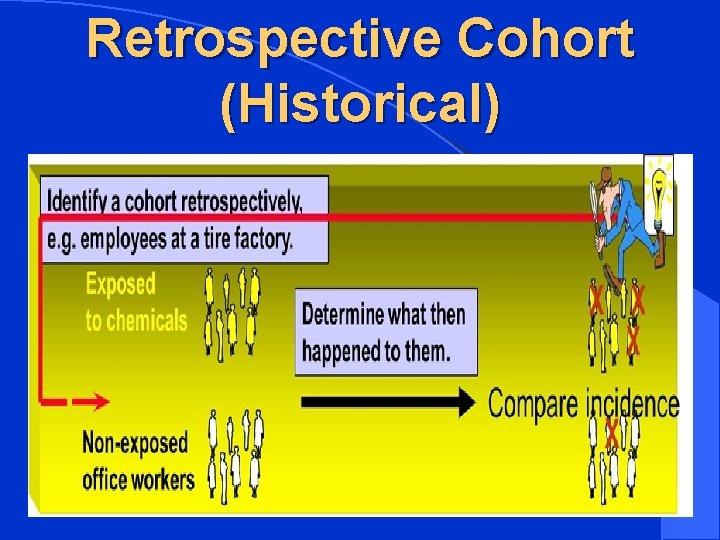
Retrospective Cohort (Historical)
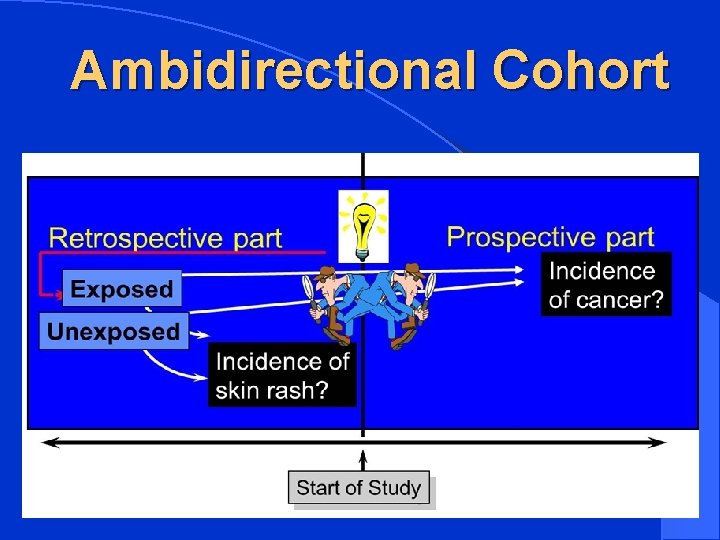
Ambidirectional Cohort

Advantages l l l l Allows study of rare exposures Can examine multiple outcomes linked to multiple exposures Can calculate Incidence, Prevalence, Relative Risk (RR) Attributable Risk (AR) and Odds Ratio (OR) directly Good for establishing temporal sequence Allows to assess natural history of disease Lower potential for bias than case-control or crosssectional studies. Can get best assessment of exposure – disease relationship

Disadvantages l Larger sample size than case-control l Long time for follow-up l Data collection is usually very expensive l Impractical for rare diseases l Impractical for diseases with long Induction period

Timeline of Milestones from the Framingham Study start of the Framingham Heart Study � 1948: l 1960: cigarette smoking found to increase risk of l heart disease � 1961: cholesterol, blood pressure, found to increase risk of heart disease � 1965: first Framingham Heart Study report on stroke � 1967: physical activity found to reduce risk of heart obesity found to increase the risk CHD and � 1970: lood pressure found to increase the risk of stroke � l 1974: diabetes found to be associated with cardiovascular disease
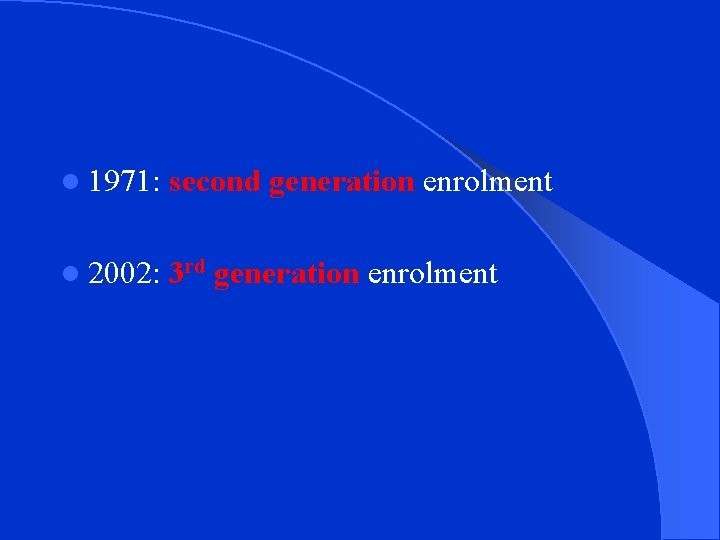
l 1971: second generation enrolment l 2002: 3 rd generation enrolment

More examples l All of us: 2018 , one million , disparity l UK biobank: recently , 500, 000 l EPIC cohort: 1998 500, 000 , Europe l Persian cohort: 2014 , 20 centers l 8 occupational , 5 newborn , 4 youth , 1 elderly

Descriptive Cohort l. Only incident rates are interested l. No comparison (association)

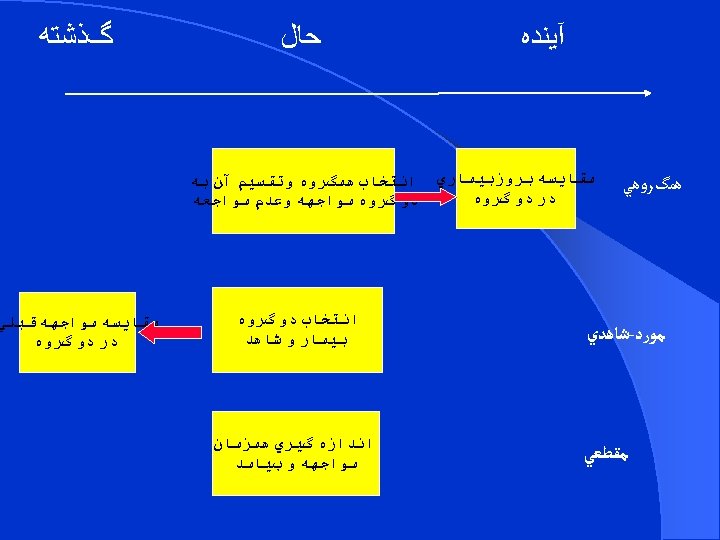
STEPS OF COHORT STUDY There are four steps in a cohort study: l Selection of study participants l Obtaining data on exposure l Follow up l Analysis

SELECTION OF STUDY PARTICIPANTS There are two basic ways to generate such groups: 1 - We can create a study population by selecting groups for inclusion in the study on the basis of whether or not they were exposed (e. g. , occupationally exposed cohorts) 2 - we can select a defined population before any of its members become exposed or before their exposures are identified.
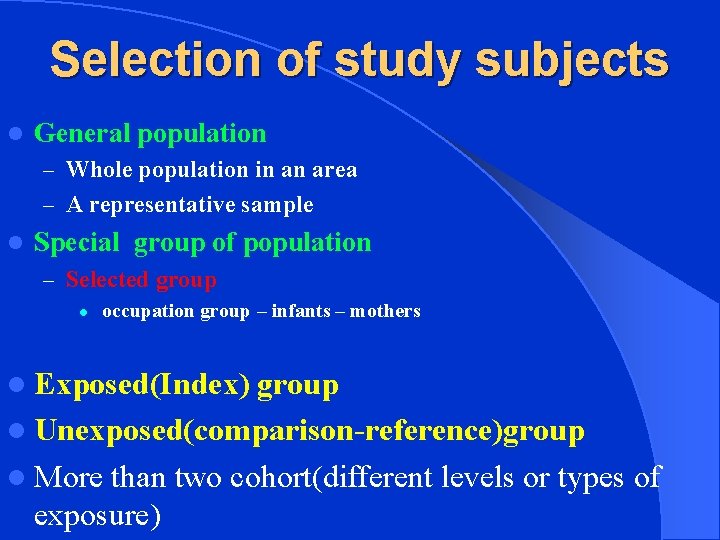
Selection of study subjects l General population – Whole population in an area – A representative sample l Special group of population – Selected group l occupation group – infants – mothers l Exposed(Index) group l Unexposed(comparison-reference)group l More than two cohort(different levels or types of exposure)
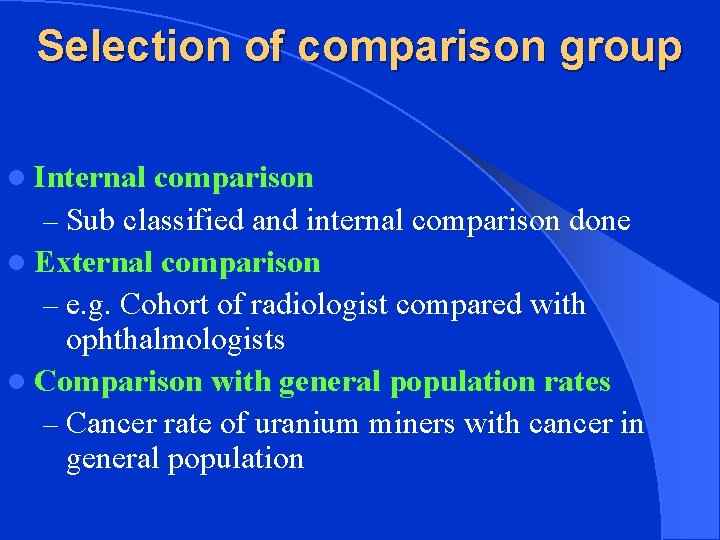
Selection of comparison group l Internal comparison – Sub classified and internal comparison done l External comparison – e. g. Cohort of radiologist compared with ophthalmologists l Comparison with general population rates – Cancer rate of uranium miners with cancer in general population

General consideration while selection of cohorts l Both the cohorts are free of the disease. l Both the groups should equally susceptible to disease l Both the groups should be comparable l Source of the exposed group: l - Common exposure : general population l - Rare exposure : high risk groups
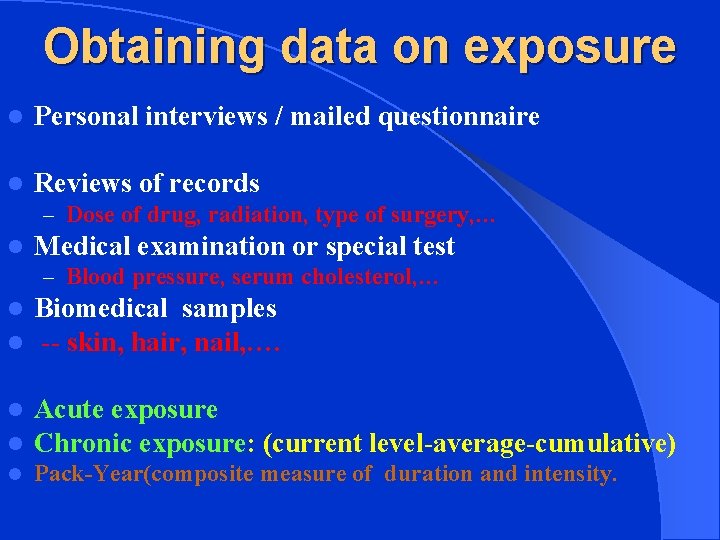
Obtaining data on exposure l Personal interviews / mailed questionnaire l Reviews of records – Dose of drug, radiation, type of surgery, … l Medical examination or special test – Blood pressure, serum cholesterol, … l l Biomedical samples -- skin, hair, nail, …. l l Acute exposure Chronic exposure: (current level-average-cumulative) l Pack-Year(composite measure of duration and intensity.

Follow-up l Follow up is the most critical part of the study l Loss to follow-up is one of the draw-back of the cohort study. l To obtain data about outcome (morbidity or death) – Mailed questionnaire, telephone calls, personal interviews – Periodic medical examination l Diagnostic criteria for the outcomes should be identical.
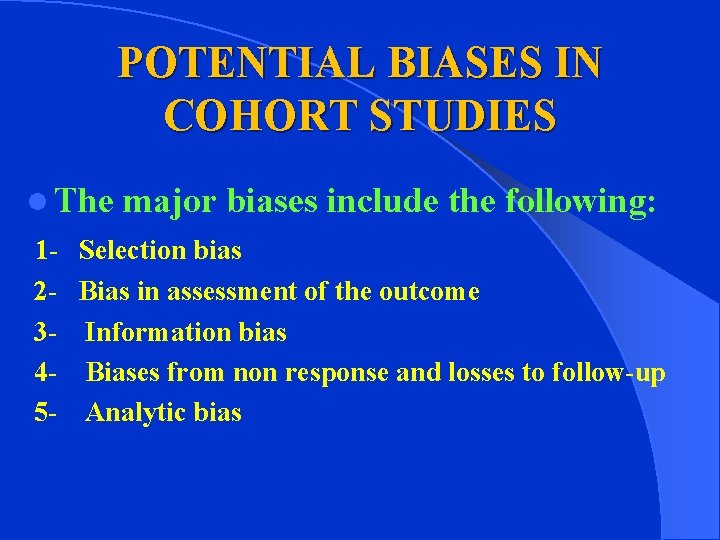
POTENTIAL BIASES IN COHORT STUDIES l The major biases include the following: 1 - Selection bias 2 - Bias in assessment of the outcome 3 - Information bias 4 - Biases from non response and losses to follow-up 5 - Analytic bias

Selection bias l Select participants into exposed and not exposed groups based on some characteristics that may affect the outcome.

Bias in assessment of the outcome l If the person who decides whether disease has developed in each subject also knows whether that subject was exposed, and if that person is aware of the hypothesis being tested, that person's judgment as to whether the disease developed may be biased by that knowledge. l This problem can be addressed by Masking (Blinding)

Information bias l Misclassify exposure status or disease status. l If the quality and extent of information obtained is different for exposed persons than for non exposed persons. l This is particularly likely to occur in historical cohort studies, in which information is obtained from past records.

Biases from non response and losses to follow-up l nonparticipation and non response can introduce major biases that can complicate the interpretation of the study findings. l Similarly, loss to follow-up can be a serious problem.

Analytic bias l As in any study, if the epidemiologists and statisticians who are analyzing the data have strong preconceptions, they may unintentionally introduce their biases into their data analyses and into their interpretation of the study findings.

ANALYSIS l. Risk l. RR(Relative Risk) l. OR(Odds Ratio) l. Incidence Density l. HR(Hazard Rate) l. AR(Attributable Risk)
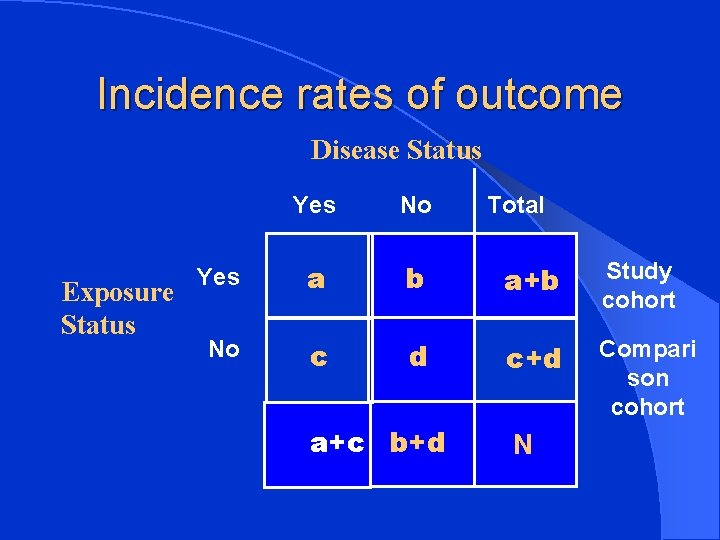
Incidence rates of outcome Disease Status Exposure Status Yes No Yes a b a+b Study cohort No c d c+d Compari son cohort a+c b+d Total N

Incidence rate l Incidence among exposed = a a+b l Incidence among non-exposed = c c+d

Estimation of risk l Relative Risk incidence of disease among exposed RR = _______________ Incidence of disease among non-exposed a/a+b = _____ c/c+d
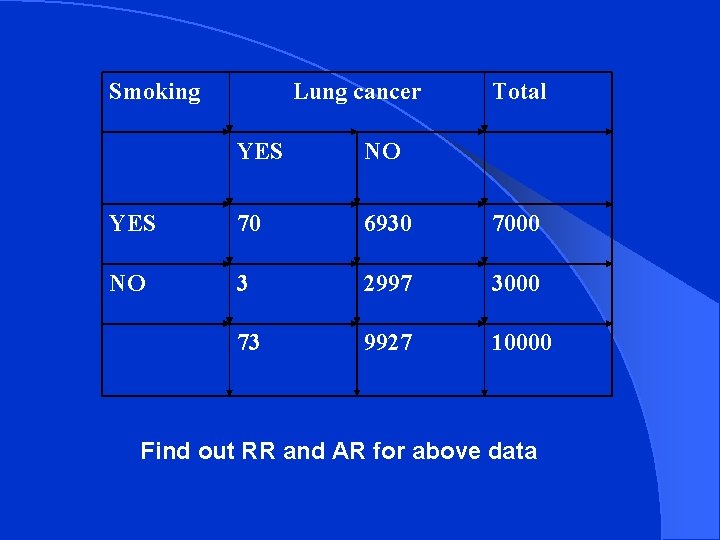
Smoking Lung cancer Total YES NO YES 70 6930 7000 NO 3 2997 3000 73 9927 10000 Find out RR and AR for above data

Estimation of Risk l Attributable Risk Incidence of disease among exposed – incidence of disease among non exposed AR = ________________ Incidence of disease among exposed a/a+b – c/c+d AR = ________ a/a+b

Incidence of lung cancer among smokers 70/7000 = 10 per 1000 l Incidence of lung cancer among non-smokers 3/3000 = 1 per thousand RR = 10 / 1 = 10 (lung cancer is 10 times more common among smokers than non smokers) AR = 10 – 1 / 10 X 100 = 90 % (90% of the cases of lung cancer among smokers are attributed to their habit of smoking) l

- Slides: 46