Epidemiology of Poliomyelitis Polio End Game Strategy Dr























- Slides: 46

Epidemiology of Poliomyelitis Polio End Game Strategy Dr Abhay S. Nirgude M. D. , DHA, PGDBEME, Faimer Fellow. Professor & Head, Community Medicine 12/23/2017 Dr Abhay Nirgude

Specific Learning Objectives • Discuss the epidemiology of poliomyelitis • Discuss the polio surveillance and polio end game strategy in India • Discuss how surveillance impacted the NPEP. • Understand potential use of polio surveillance model in control of other diseases. 12/23/2017 Dr Abhay Nirgude

12/23/2017 Dr Abhay Nirgude

Polio Cases & SIAs 12/23/2017 Dr Abhay Nirgude

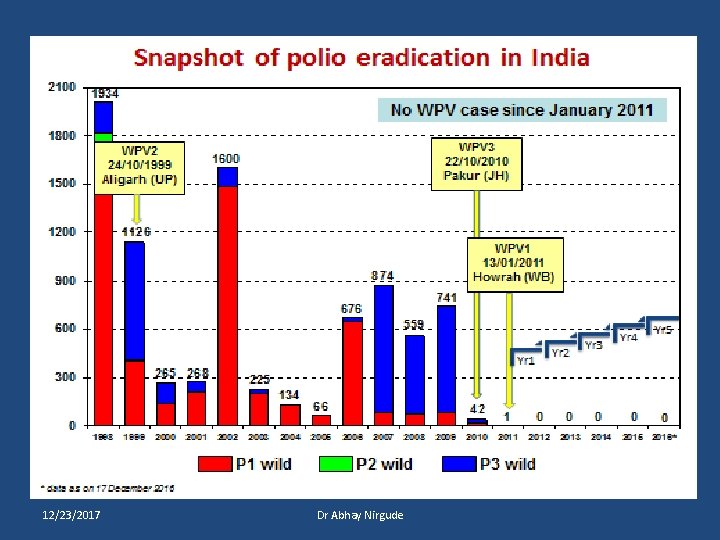
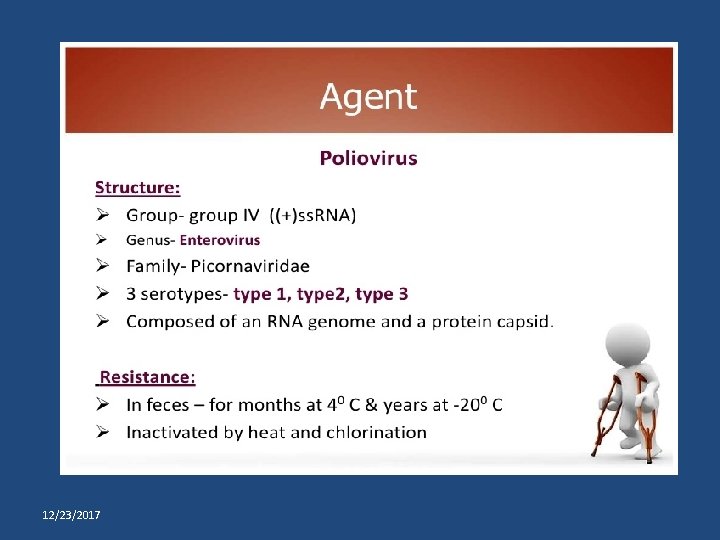
Last wild poliovirus cases by type, India WPV 2 24/10/1999 Aligarh (UP) WPV 3 22/10/2010 Pakur (JH) WPV 1 13/01/2011 Howrah (WB) 12/23/2017 Dr Abhay Nirgude

12/23/2017

12/23/2017

12/23/2017

12/23/2017

12/23/2017

12/23/2017

12/23/2017

12/23/2017

12/23/2017

12/23/2017

Prevention 12/23/2017 Dr Abhay Nirgude

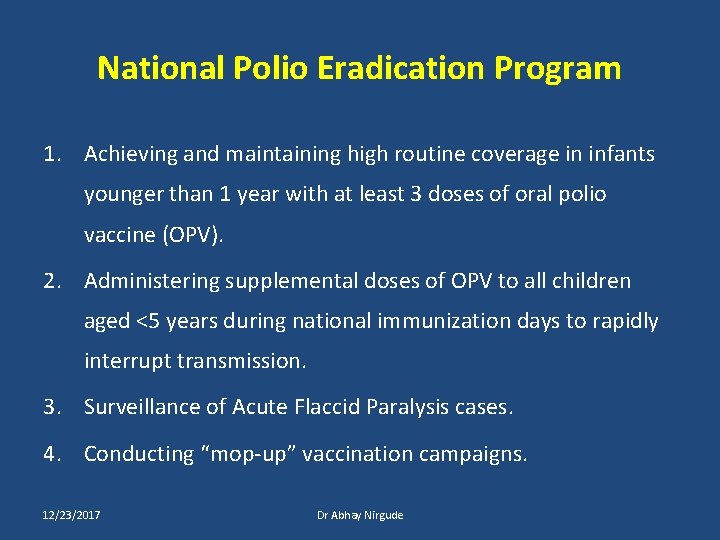
National Polio Eradication Program 1. Achieving and maintaining high routine coverage in infants younger than 1 year with at least 3 doses of oral polio vaccine (OPV). 2. Administering supplemental doses of OPV to all children aged <5 years during national immunization days to rapidly interrupt transmission. 3. Surveillance of Acute Flaccid Paralysis cases. 4. Conducting “mop-up” vaccination campaigns. 12/23/2017 Dr Abhay Nirgude

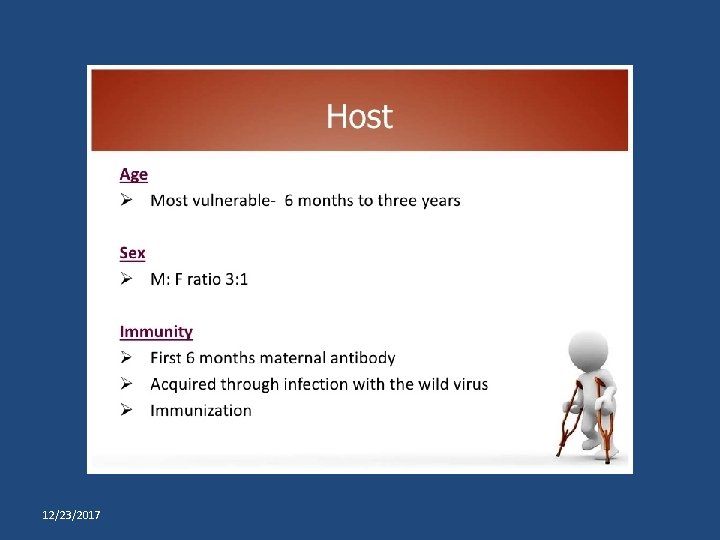
Salk versus Sabin vaccine IPV (Salk) OPV (Sabin) killed formolised virus Given SC or IM or ID Induces circulating antibodies, but not local (intestinal immunity) Prevents paralysis but does not prevent reinfection Not useful in controlling epidemics More difficult to manufacture and is relatively costly Does not require stringent conditions during storage and transportation. Has a longer shelf life. live attenuated virus given orally immunity is both humoral and intestinal. induces antibody quickly Prevents paralysis and prevents reinfection Can be effectively used in controlling epidemics. Easy to manufacture and is cheaper Requires to be stored and transported at subzero temperatures, and is damaged easily. 12/23/2017 Dr Abhay Nirgude

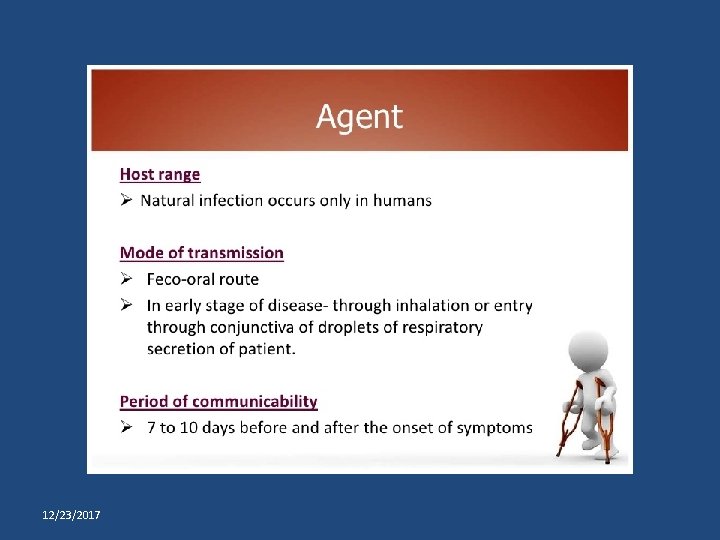
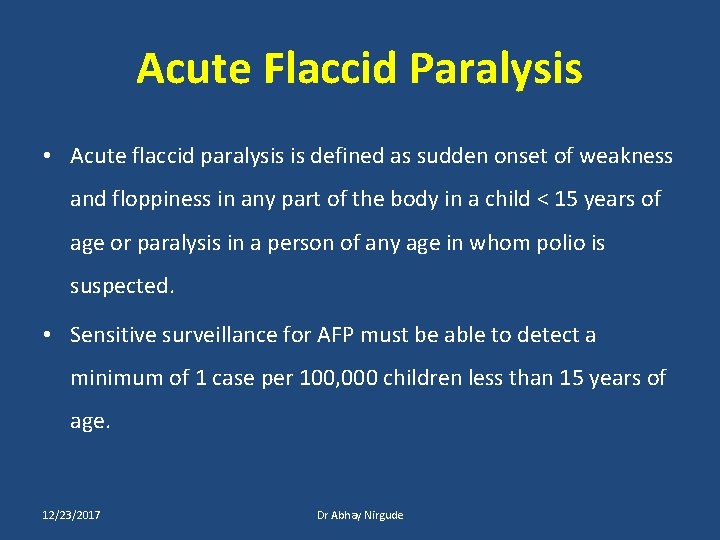
Acute Flaccid Paralysis • Acute flaccid paralysis is defined as sudden onset of weakness and floppiness in any part of the body in a child < 15 years of age or paralysis in a person of any age in whom polio is suspected. • Sensitive surveillance for AFP must be able to detect a minimum of 1 case per 100, 000 children less than 15 years of age. 12/23/2017 Dr Abhay Nirgude

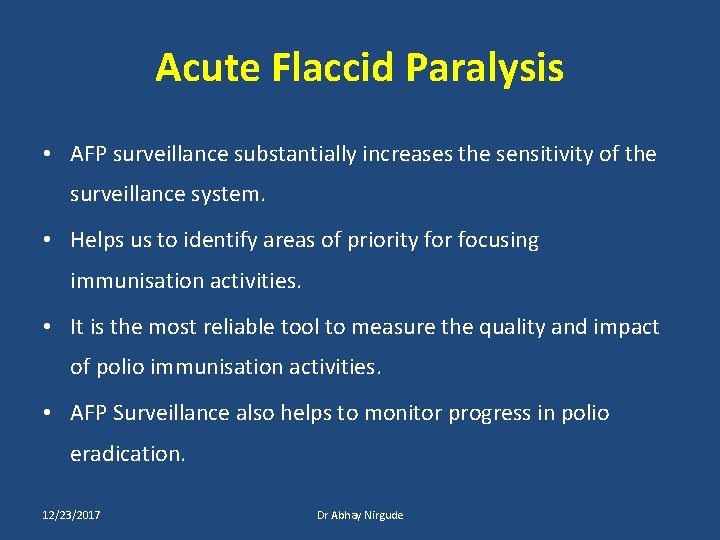
Acute Flaccid Paralysis • AFP surveillance substantially increases the sensitivity of the surveillance system. • Helps us to identify areas of priority for focusing immunisation activities. • It is the most reliable tool to measure the quality and impact of polio immunisation activities. • AFP Surveillance also helps to monitor progress in polio eradication. 12/23/2017 Dr Abhay Nirgude

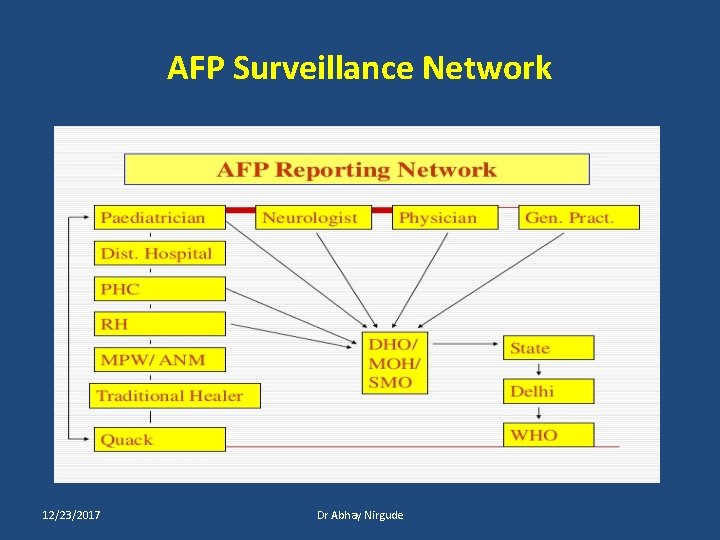
AFP Surveillance Network 12/23/2017 Dr Abhay Nirgude

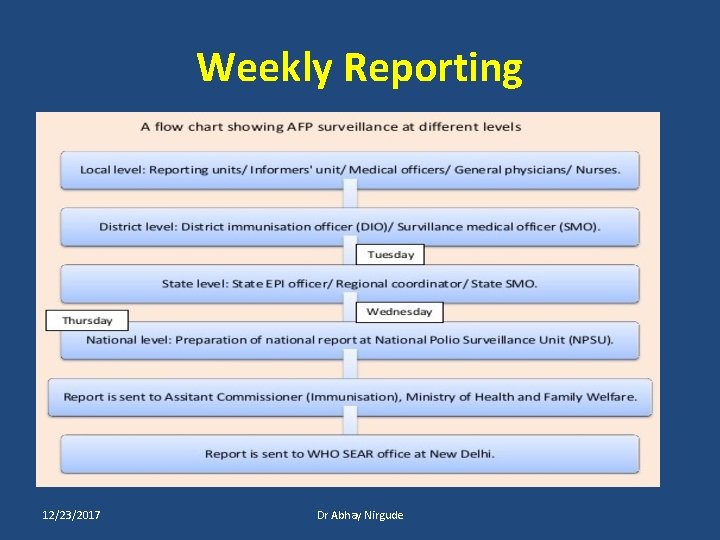
Weekly Reporting 12/23/2017 Dr Abhay Nirgude

AFP Surveillance AFP Case Investigation 12/23/2017 Visit to Reporting Unit Dr Abhay Nirgude

STOOL COLLECTION, STORAGE , TRANSPORT. • LRF • Adequate Stool. – 2 Specimens, 24 Hours Apart. – 8 gms. – Within 14 Days of Paralysis Onset & with proper Cold Chain • Reverse Cold Chain. 12/23/2017 Dr Abhay Nirgude

Surveillance System Performance Indicators • Non – Polio AFP Rate > 2. 0 (India) • Adequate Stool Samples > 80% • Timeliness of Reporting > 80% • Reported AFP cases investigated within 48 hours of notification (target >80%). • Timeliness of weekly reporting (target >80%) 12/23/2017 Dr Abhay Nirgude

Surveillance System Performance Indicators • Completeness of weekly reporting (target >90%). • Stool specimens reaching a WHO accredited laboratory within 72 hours of being sent ( >80%). • Stool specimens reaching laboratory in good condition (target >80%). • Stool specimens with a turn around time <28 days (>80%) 12/23/2017 Dr Abhay Nirgude

Identify Areas/ Population at risk • Real time and credible data. • Tailored strategies to ensure coverage • Migrant population • Vulnerable population • High risk areas 12/23/2017 Dr Abhay Nirgude

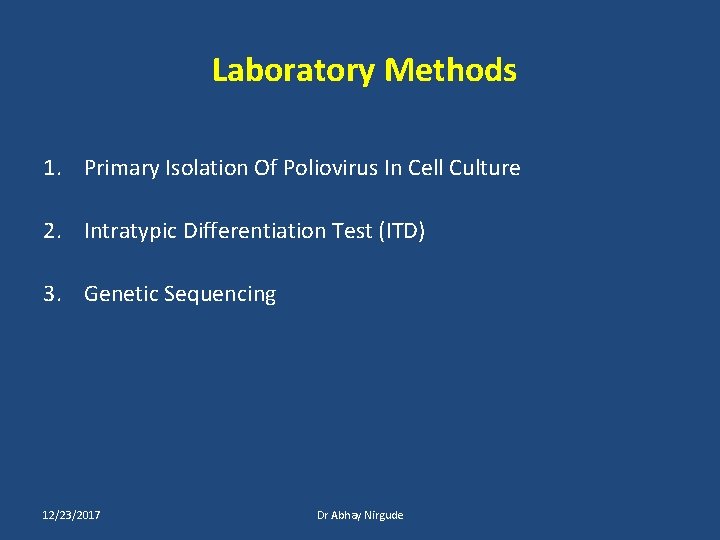
Laboratory Methods 1. Primary Isolation Of Poliovirus In Cell Culture 2. Intratypic Differentiation Test (ITD) 3. Genetic Sequencing 12/23/2017 Dr Abhay Nirgude

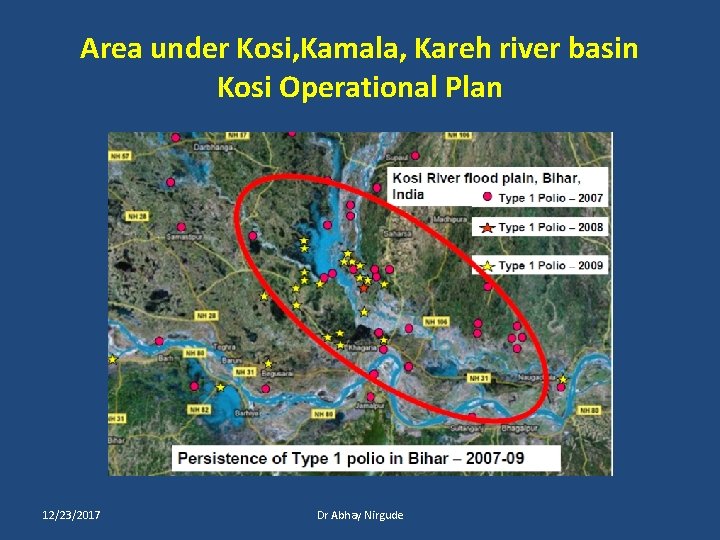
Area under Kosi, Kamala, Kareh river basin Kosi Operational Plan 12/23/2017 Dr Abhay Nirgude

Kosi Operational Plan • Kosi operational group • Head : - Principal secretary HFW • Social Mobilization : - UNICEF • Technical & Operational Inputs: - WHO-NPSP • Key field personals : - Health department. 12/23/2017 Dr Abhay Nirgude

Development of Underserved Strategy 12/23/2017 Dr Abhay Nirgude

Influence Policy Makers • Advocacy. • Facilitating communication. • Resolving disputes. • Liasoning with stakeholders at all levels. 12/23/2017 Dr Abhay Nirgude

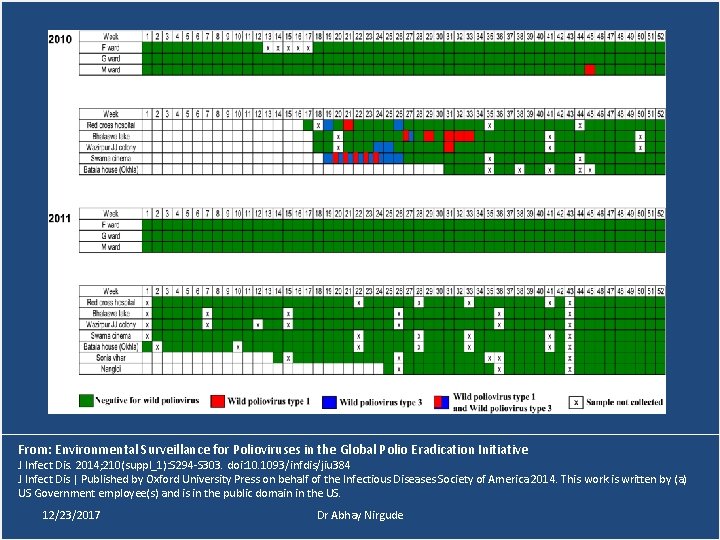
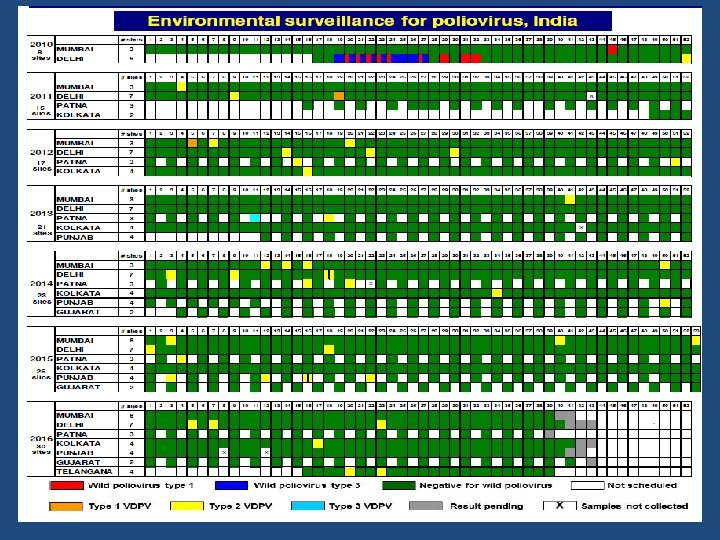
Environmental Surveillance • Detect poliovirus circulation by means of sewage sampling. • Polioviruses (WPV & VDPV) are sequenced to characterize their genetic relationship to polioviruses isolated from other environmental samples and from AFP cases. • The combination of AFP and ES data are then used to identify areas of increased risk and to specifically target vaccination strategies to better reach susceptible populations. 12/23/2017 Dr Abhay Nirgude

Environmental Surveillance • On at least a few occasions, WPVs of the same genetic lineage were isolated from sewage samples before the detection of a paralytic case through AFP surveillance. • Genetic sequencing may allow the ability to trace the origin of polioviruses to other circulating polioviruses with similar sequences. 12/23/2017 Dr Abhay Nirgude

Environmental Surveillance Sites • Mumbai 2001 • Delhi 2010 • Patna & Kolkata 2011 • Punjab & Gujarat 2013 • Telangana & Uttar Pradesh 12/23/2017 Dr Abhay Nirgude

From: Environmental Surveillance for Polioviruses in the Global Polio Eradication Initiative J Infect Dis. 2014; 210(suppl_1): S 294 -S 303. doi: 10. 1093/infdis/jiu 384 J Infect Dis | Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2014. This work is written by (a) US Government employee(s) and is in the public domain in the US. 12/23/2017 Dr Abhay Nirgude

12/23/2017 Dr Abhay Nirgude

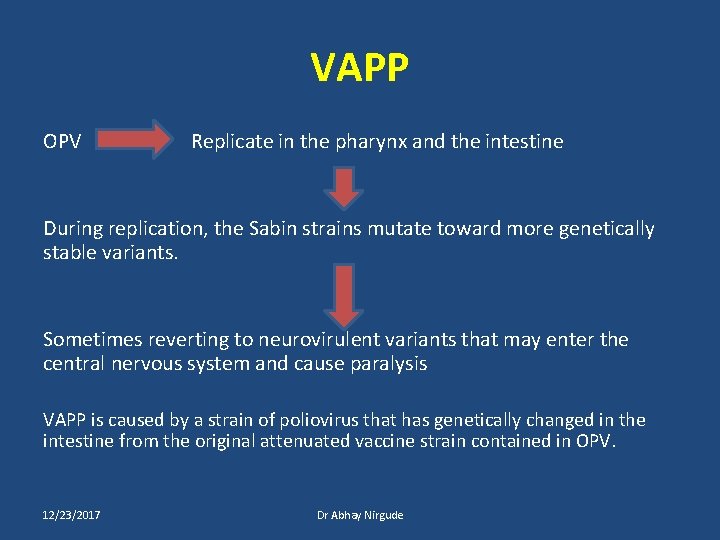
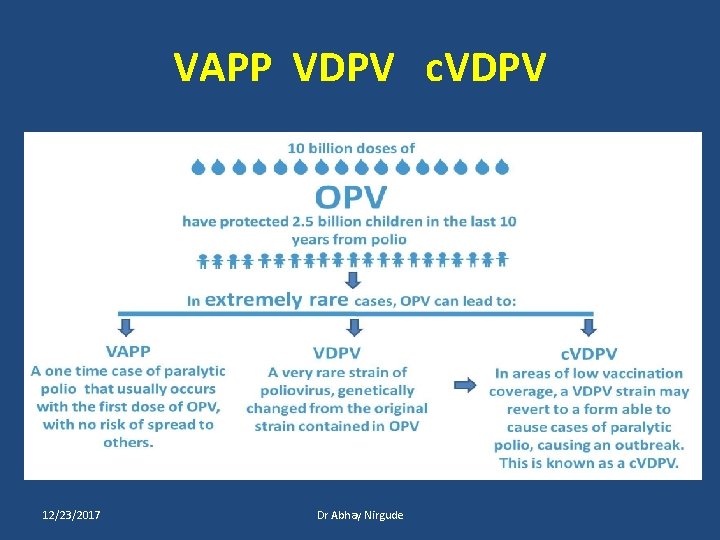
VAPP OPV Replicate in the pharynx and the intestine During replication, the Sabin strains mutate toward more genetically stable variants. Sometimes reverting to neurovirulent variants that may enter the central nervous system and cause paralysis VAPP is caused by a strain of poliovirus that has genetically changed in the intestine from the original attenuated vaccine strain contained in OPV. 12/23/2017 Dr Abhay Nirgude

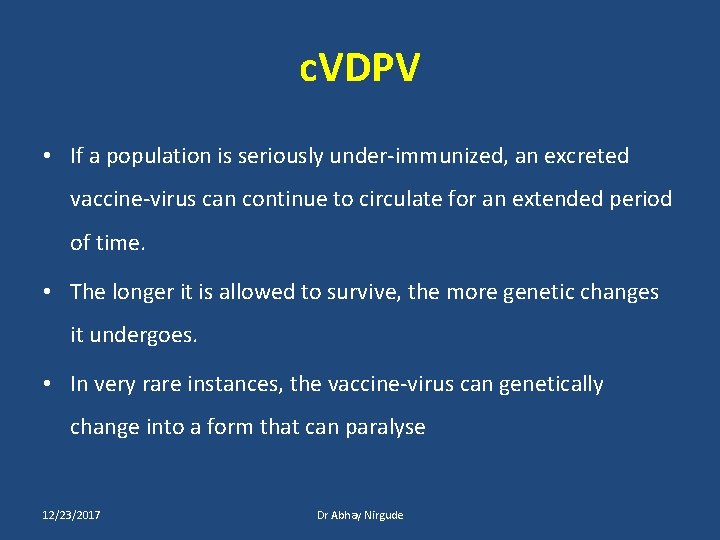
c. VDPV • If a population is seriously under-immunized, an excreted vaccine-virus can continue to circulate for an extended period of time. • The longer it is allowed to survive, the more genetic changes it undergoes. • In very rare instances, the vaccine-virus can genetically change into a form that can paralyse 12/23/2017 Dr Abhay Nirgude

VAPP VDPV c. VDPV 12/23/2017 Dr Abhay Nirgude

Objectives of the Polio Eradication & Endgame Strategic Plan 2013 -2018 1 • Detect and interrupt all poliovirus transmission 2 • Strengthen immunization systems, introduce inactivated polio vaccine (IPV) and withdraw oral polio vaccines (OPV) 3 • Contain poliovirus and certify interruption of transmission 4 • Plan polio’s legacy 12/23/2017 Dr Abhay Nirgude

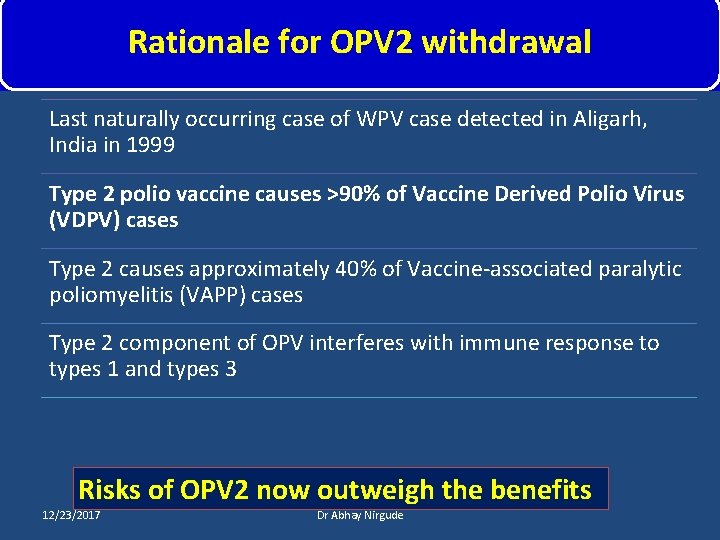
Rationale for OPV 2 withdrawal Last naturally occurring case of WPV case detected in Aligarh, India in 1999 Type 2 polio vaccine causes >90% of Vaccine Derived Polio Virus (VDPV) cases Type 2 causes approximately 40% of Vaccine-associated paralytic poliomyelitis (VAPP) cases Type 2 component of OPV interferes with immune response to types 1 and types 3 Risks of OPV 2 now outweigh the benefits 12/23/2017 Dr Abhay Nirgude

Risks associated with OPV type 2 withdrawal • Withdrawal of OPV type 2 will leave a gap in population immunity against type 2 poliovirus • Increased risk of outbreaks due to type 2 poliovirus following reintroduction • Re-introduction could occur if: – c. VDPV type 2 emerged during or shortly after OPV type 2 withdrawal – Importation of c. VDPVs occurs – Break in bio-containment process in laboratories storing viruses. Risks associated with OPV type 2 withdrawal can be mitigated

immunity against type 2 polio a) Emphasis on routine immunization strengthening - raising coverage critical for achieving the endgame b) Introduce IPV 12/23/2017 Dr Abhay Nirgude

Potential Use In Other Diseases • National Public Health surveillance Project: Technical support : - 1. VPD’s 2. Outbreak management IDSP 3. Neglected tropical diseases • Measles • Zika Virus 12/23/2017 Dr Abhay Nirgude

Thank You 12/23/2017 Dr Abhay Nirgude
 Parasite
Parasite Types of poliomyelitis
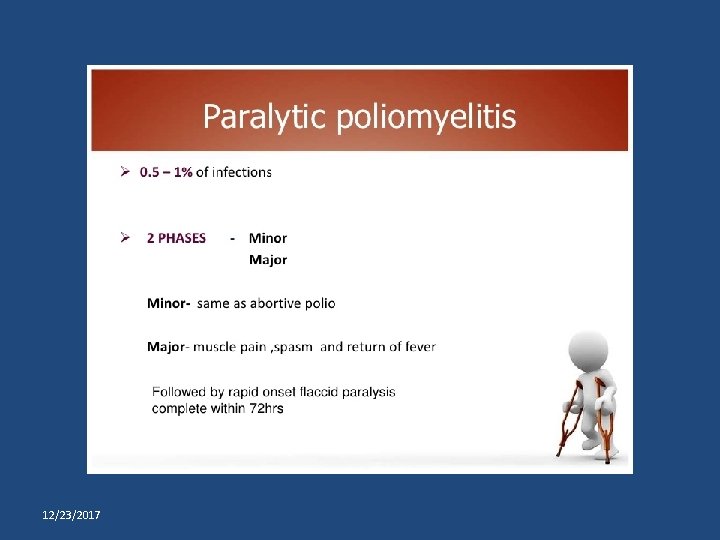
Types of poliomyelitis Primary prevention of poliomyelitis
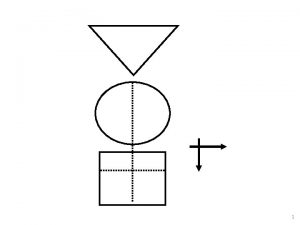
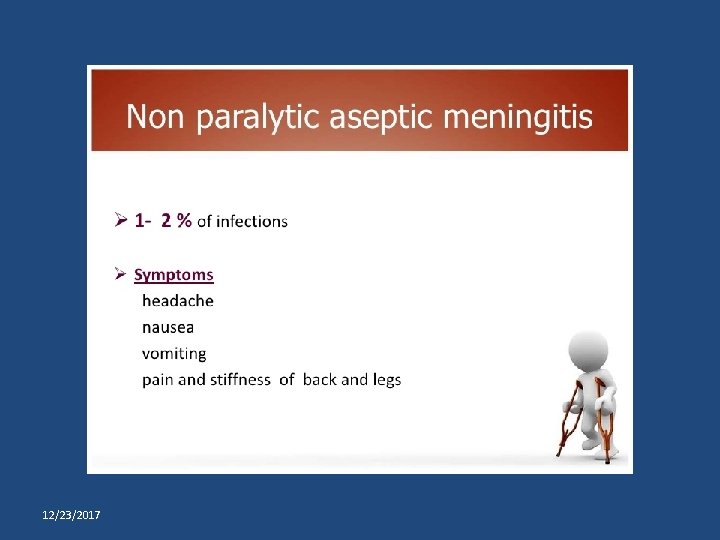
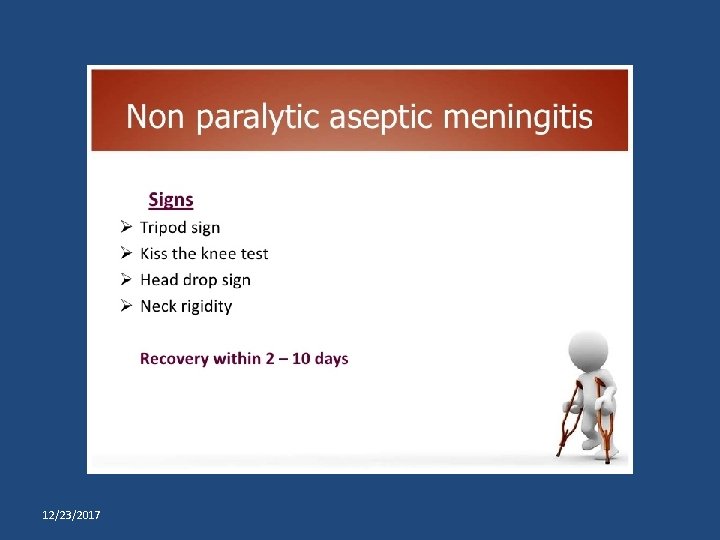
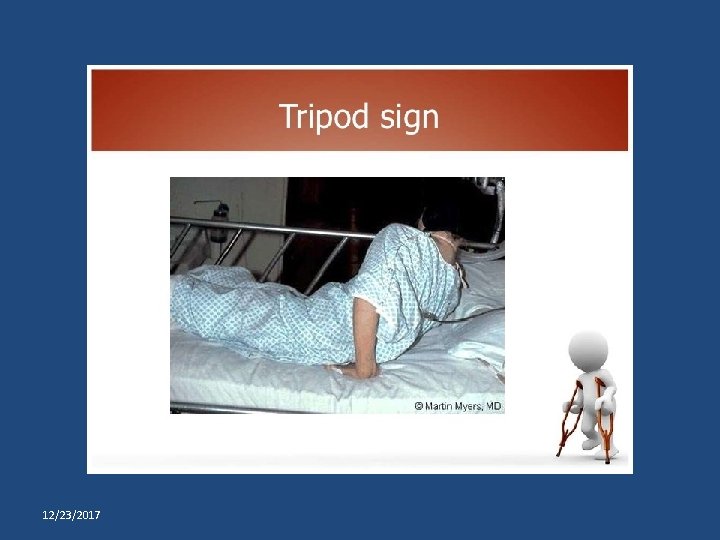
Primary prevention of poliomyelitis Tripod sign polio
Tripod sign polio Prevention and control of poliomyelitis
Prevention and control of poliomyelitis Poliomyelitis
Poliomyelitis Prevention and control of poliomyelitis
Prevention and control of poliomyelitis Post polio syndrome
Post polio syndrome What is polio disease
What is polio disease Kernig
Kernig Hans tolzin wikipedia
Hans tolzin wikipedia Cell response
Cell response Polio vaccine acronym
Polio vaccine acronym Porto polio
Porto polio Brack plaques radium
Brack plaques radium Polio medical term color
Polio medical term color Polio
Polio Polio plus
Polio plus Polio
Polio Risiko relatif dan odds ratio
Risiko relatif dan odds ratio Advantages and disadvantages of nutritional epidemiology
Advantages and disadvantages of nutritional epidemiology Logistic regression epidemiology
Logistic regression epidemiology Prevalence calculation formula
Prevalence calculation formula Ecological study design
Ecological study design Attack rate epidemiology formula
Attack rate epidemiology formula Difference between descriptive and analytic epidemiology
Difference between descriptive and analytic epidemiology Epidemiology person place time
Epidemiology person place time Descriptive vs analytical epidemiology
Descriptive vs analytical epidemiology How to calculate incidence rate example
How to calculate incidence rate example Pros and cons of cross sectional study
Pros and cons of cross sectional study Temporal relationship epidemiology example
Temporal relationship epidemiology example Attack rate calculation
Attack rate calculation Gate frame epidemiology
Gate frame epidemiology Biological plausibility example
Biological plausibility example Percentage defination
Percentage defination Defination of epidemiology
Defination of epidemiology Endemic definition in community health nursing
Endemic definition in community health nursing What is descriptive study in epidemiology
What is descriptive study in epidemiology Spurious association
Spurious association Field epidemiology ppt
Field epidemiology ppt Bhisma murti
Bhisma murti Certification board of infection control and epidemiology
Certification board of infection control and epidemiology Gordon epidemiology
Gordon epidemiology Epidemiology kept simple
Epidemiology kept simple Diabetic ketoacidosis epidemiology
Diabetic ketoacidosis epidemiology Distribution in epidemiology
Distribution in epidemiology Confounding vs effect modification
Confounding vs effect modification