EPA www epa gov In Vitro Assessment of
- Slides: 2

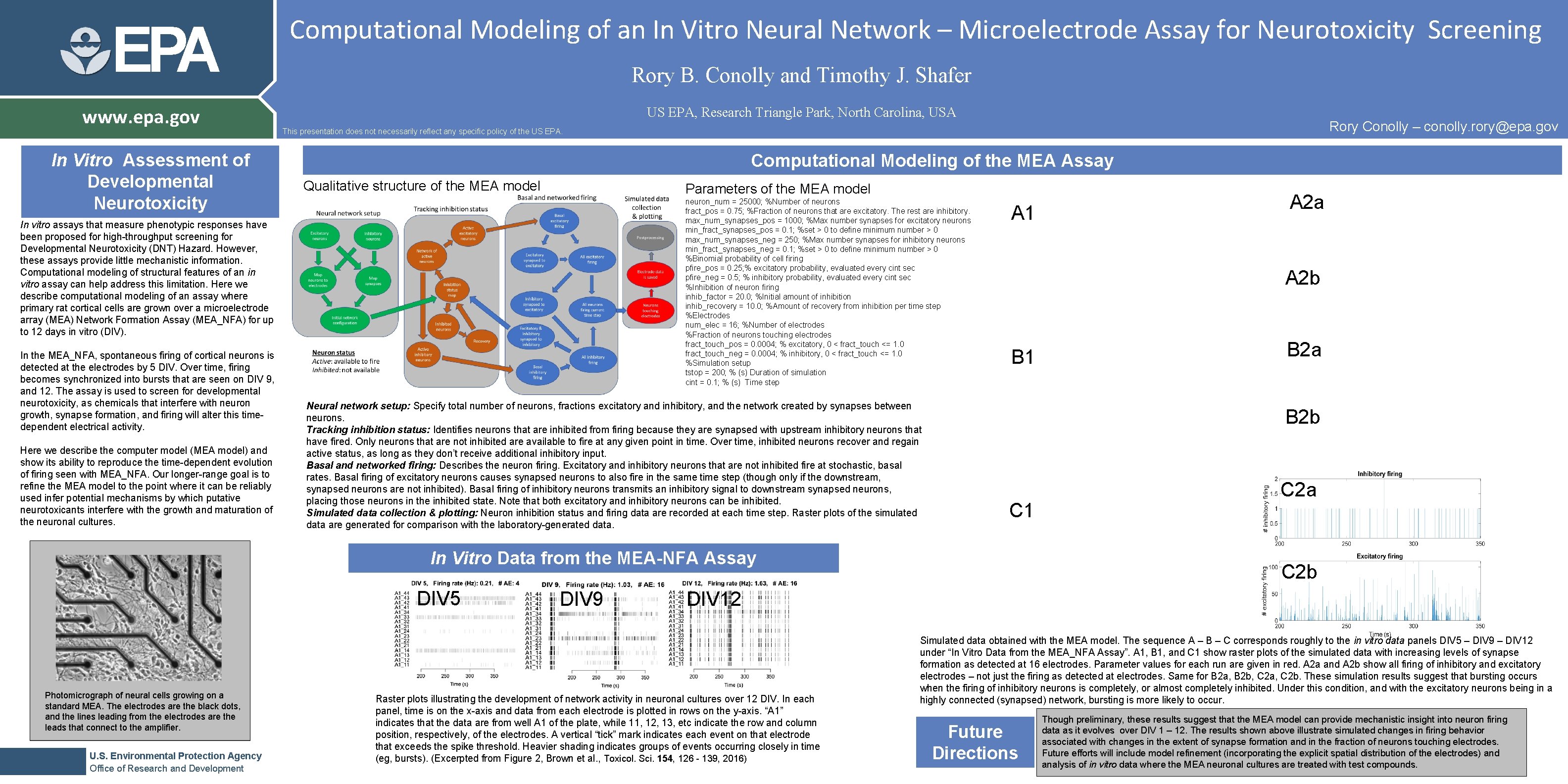
EPA www. epa. gov In Vitro Assessment of Developmental Neurotoxicity Computational Modeling of an In Vitro Neural Network – Microelectrode Assay for Neurotoxicity Screening Rory B. Conolly and Timothy J. Shafer US EPA, Research Triangle Park, North Carolina, USA Computational Modeling of the MEA Assay Qualitative structure of the MEA model Parameters of the MEA model neuron_num = 25000; %Number of neurons fract_pos = 0. 75; %Fraction of neurons that are excitatory. The rest are inhibitory. max_num_synapses_pos = 1000; %Max number synapses for excitatory neurons min_fract_synapses_pos = 0. 1; %set > 0 to define minimum number > 0 max_num_synapses_neg = 250; %Max number synapses for inhibitory neurons min_fract_synapses_neg = 0. 1; %set > 0 to define minimum number > 0 %Binomial probability of cell firing pfire_pos = 0. 25; % excitatory probability, evaluated every cint sec pfire_neg = 0. 5; % inhibitory probability, evaluated every cint sec %Inhibition of neuron firing inhib_factor = 20. 0; %Initial amount of inhibition inhib_recovery = 10. 0; %Amount of recovery from inhibition per time step %Electrodes num_elec = 16; %Number of electrodes %Fraction of neurons touching electrodes fract_touch_pos = 0. 0004; % excitatory, 0 < fract_touch <= 1. 0 fract_touch_neg = 0. 0004; % inhibitory, 0 < fract_touch <= 1. 0 %Simulation setup tstop = 200; % (s) Duration of simulation cint = 0. 1; % (s) Time step In vitro assays that measure phenotypic responses have been proposed for high-throughput screening for Developmental Neurotoxicity (DNT) Hazard. However, these assays provide little mechanistic information. Computational modeling of structural features of an in vitro assay can help address this limitation. Here we describe computational modeling of an assay where primary rat cortical cells are grown over a microelectrode array (MEA) Network Formation Assay (MEA_NFA) for up to 12 days in vitro (DIV). In the MEA_NFA, spontaneous firing of cortical neurons is detected at the electrodes by 5 DIV. Over time, firing becomes synchronized into bursts that are seen on DIV 9, and 12. The assay is used to screen for developmental neurotoxicity, as chemicals that interfere with neuron growth, synapse formation, and firing will alter this timedependent electrical activity. Here we describe the computer model (MEA model) and show its ability to reproduce the time-dependent evolution of firing seen with MEA_NFA. Our longer-range goal is to refine the MEA model to the point where it can be reliably used infer potential mechanisms by which putative neurotoxicants interfere with the growth and maturation of the neuronal cultures. Rory Conolly – conolly. rory@epa. gov This presentation does not necessarily reflect any specific policy of the US EPA. Neural network setup: Specify total number of neurons, fractions excitatory and inhibitory, and the network created by synapses between neurons. Tracking inhibition status: Identifies neurons that are inhibited from firing because they are synapsed with upstream inhibitory neurons that have fired. Only neurons that are not inhibited are available to fire at any given point in time. Over time, inhibited neurons recover and regain active status, as long as they don’t receive additional inhibitory input. Basal and networked firing: Describes the neuron firing. Excitatory and inhibitory neurons that are not inhibited fire at stochastic, basal rates. Basal firing of excitatory neurons causes synapsed neurons to also fire in the same time step (though only if the downstream, synapsed neurons are not inhibited). Basal firing of inhibitory neurons transmits an inhibitory signal to downstream synapsed neurons, placing those neurons in the inhibited state. Note that both excitatory and inhibitory neurons can be inhibited. Simulated data collection & plotting: Neuron inhibition status and firing data are recorded at each time step. Raster plots of the simulated data are generated for comparison with the laboratory-generated data. A 1 A 2 b B 1 Photomicrograph of neural cells growing on a standard MEA. The electrodes are the black dots, and the lines leading from the electrodes are the leads that connect to the amplifier. U. S. Environmental Protection Agency Office of Research and Development DIV 9 B 2 a B 2 b C 1 In Vitro Data from the MEA-NFA Assay DIV 5 A 2 a C 2 b DIV 12 Raster plots illustrating the development of network activity in neuronal cultures over 12 DIV. In each panel, time is on the x-axis and data from each electrode is plotted in rows on the y-axis. “A 1” indicates that the data are from well A 1 of the plate, while 11, 12, 13, etc indicate the row and column position, respectively, of the electrodes. A vertical “tick” mark indicates each event on that electrode that exceeds the spike threshold. Heavier shading indicates groups of events occurring closely in time (eg, bursts). (Excerpted from Figure 2, Brown et al. , Toxicol. Sci. 154, 126 - 139, 2016) Simulated data obtained with the MEA model. The sequence A – B – C corresponds roughly to the in vitro data panels DIV 5 – DIV 9 – DIV 12 under “In Vitro Data from the MEA_NFA Assay”. A 1, B 1, and C 1 show raster plots of the simulated data with increasing levels of synapse formation as detected at 16 electrodes. Parameter values for each run are given in red. A 2 a and A 2 b show all firing of inhibitory and excitatory electrodes – not just the firing as detected at electrodes. Same for B 2 a, B 2 b, C 2 a, C 2 b. These simulation results suggest that bursting occurs when the firing of inhibitory neurons is completely, or almost completely inhibited. Under this condition, and with the excitatory neurons being in a highly connected (synapsed) network, bursting is more likely to occur. Future Directions Though preliminary, these results suggest that the MEA model can provide mechanistic insight into neuron firing data as it evolves over DIV 1 – 12. The results shown above illustrate simulated changes in firing behavior associated with changes in the extent of synapse formation and in the fraction of neurons touching electrodes. Future efforts will include model refinement (incorporating the explicit spatial distribution of the electrodes) and analysis of in vitro data where the MEA neuronal cultures are treated with test compounds.

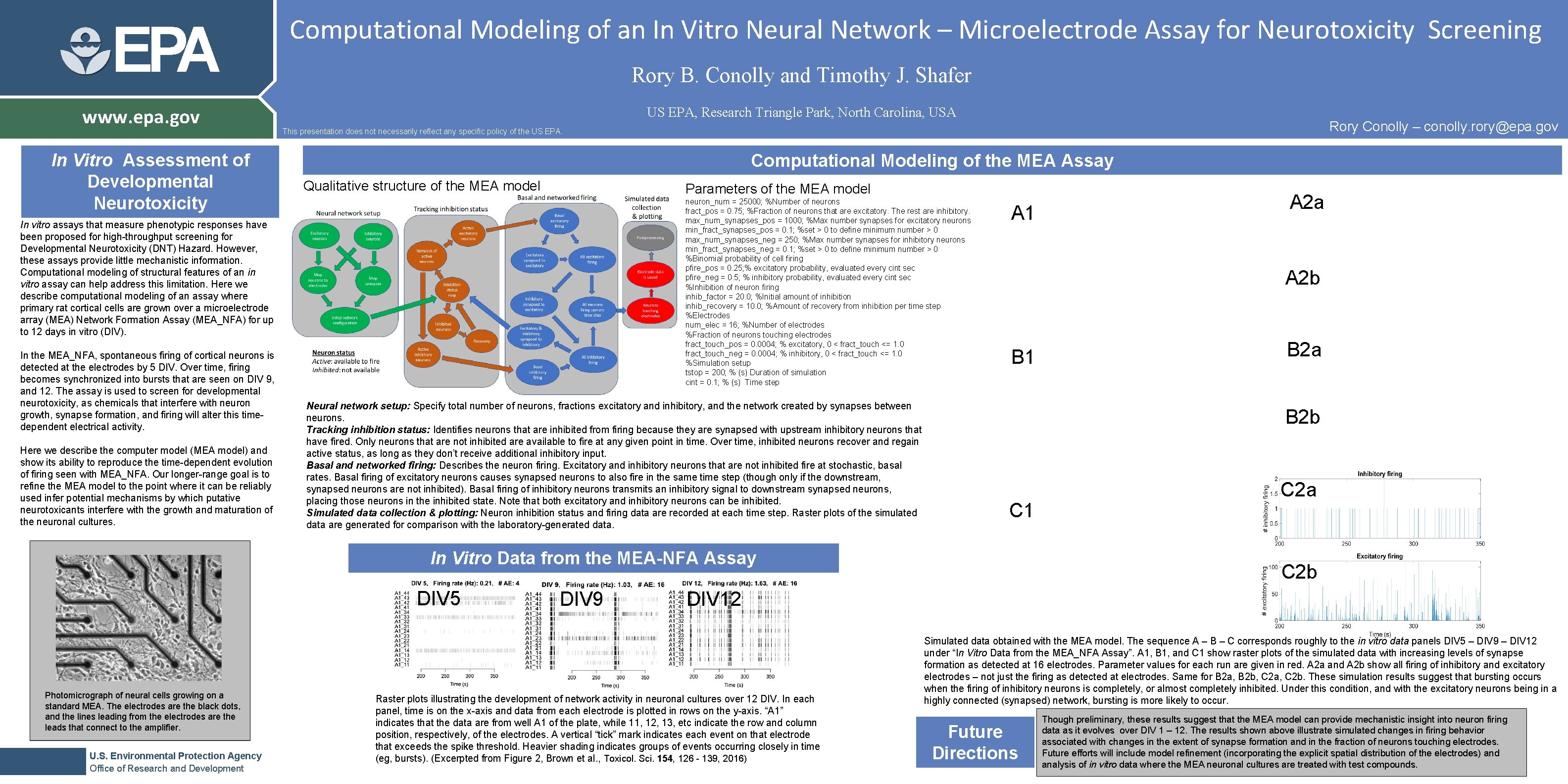
EPA www. epa. gov In Vitro Assessment of Developmental Neurotoxicity Computational Modeling of an In Vitro Neural Network – Microelectrode Assay for Neurotoxicity Screening Rory B. Conolly and Timothy J. Shafer US EPA, Research Triangle Park, North Carolina, USA Computational Modeling of the MEA Assay Qualitative structure of the MEA model Parameters of the MEA model neuron_num = 25000; %Number of neurons fract_pos = 0. 75; %Fraction of neurons that are excitatory. The rest are inhibitory. max_num_synapses_pos = 1000; %Max number synapses for excitatory neurons min_fract_synapses_pos = 0. 1; %set > 0 to define minimum number > 0 max_num_synapses_neg = 250; %Max number synapses for inhibitory neurons min_fract_synapses_neg = 0. 1; %set > 0 to define minimum number > 0 %Binomial probability of cell firing pfire_pos = 0. 25; % excitatory probability, evaluated every cint sec pfire_neg = 0. 5; % inhibitory probability, evaluated every cint sec %Inhibition of neuron firing inhib_factor = 20. 0; %Initial amount of inhibition inhib_recovery = 10. 0; %Amount of recovery from inhibition per time step %Electrodes num_elec = 16; %Number of electrodes %Fraction of neurons touching electrodes fract_touch_pos = 0. 0004; % excitatory, 0 < fract_touch <= 1. 0 fract_touch_neg = 0. 0004; % inhibitory, 0 < fract_touch <= 1. 0 %Simulation setup tstop = 200; % (s) Duration of simulation cint = 0. 1; % (s) Time step In vitro assays that measure phenotypic responses have been proposed for high-throughput screening for Developmental Neurotoxicity (DNT) Hazard. However, these assays provide little mechanistic information. Computational modeling of structural features of an in vitro assay can help address this limitation. Here we describe computational modeling of an assay where primary rat cortical cells are grown over a microelectrode array (MEA) Network Formation Assay (MEA_NFA) for up to 12 days in vitro (DIV). In the MEA_NFA, spontaneous firing of cortical neurons is detected at the electrodes by 5 DIV. Over time, firing becomes synchronized into bursts that are seen on DIV 9, and 12. The assay is used to screen for developmental neurotoxicity, as chemicals that interfere with neuron growth, synapse formation, and firing will alter this timedependent electrical activity. Here we describe the computer model (MEA model) and show its ability to reproduce the time-dependent evolution of firing seen with MEA_NFA. Our longer-range goal is to refine the MEA model to the point where it can be reliably used infer potential mechanisms by which putative neurotoxicants interfere with the growth and maturation of the neuronal cultures. Rory Conolly – conolly. rory@epa. gov This presentation does not necessarily reflect any specific policy of the US EPA. Neural network setup: Specify total number of neurons, fractions excitatory and inhibitory, and the network created by synapses between neurons. Tracking inhibition status: Identifies neurons that are inhibited from firing because they are synapsed with upstream inhibitory neurons that have fired. Only neurons that are not inhibited are available to fire at any given point in time. Over time, inhibited neurons recover and regain active status, as long as they don’t receive additional inhibitory input. Basal and networked firing: Describes the neuron firing. Excitatory and inhibitory neurons that are not inhibited fire at stochastic, basal rates. Basal firing of excitatory neurons causes synapsed neurons to also fire in the same time step (though only if the downstream, synapsed neurons are not inhibited). Basal firing of inhibitory neurons transmits an inhibitory signal to downstream synapsed neurons, placing those neurons in the inhibited state. Note that both excitatory and inhibitory neurons can be inhibited. Simulated data collection & plotting: Neuron inhibition status and firing data are recorded at each time step. Raster plots of the simulated data are generated for comparison with the laboratory-generated data. A 1 A 2 b B 1 Photomicrograph of neural cells growing on a standard MEA. The electrodes are the black dots, and the lines leading from the electrodes are the leads that connect to the amplifier. U. S. Environmental Protection Agency Office of Research and Development DIV 9 B 2 a B 2 b C 1 In Vitro Data from the MEA-NFA Assay DIV 5 A 2 a C 2 b DIV 12 Raster plots illustrating the development of network activity in neuronal cultures over 12 DIV. In each panel, time is on the x-axis and data from each electrode is plotted in rows on the y-axis. “A 1” indicates that the data are from well A 1 of the plate, while 11, 12, 13, etc indicate the row and column position, respectively, of the electrodes. A vertical “tick” mark indicates each event on that electrode that exceeds the spike threshold. Heavier shading indicates groups of events occurring closely in time (eg, bursts). (Excerpted from Figure 2, Brown et al. , Toxicol. Sci. 154, 126 - 139, 2016) Simulated data obtained with the MEA model. The sequence A – B – C corresponds roughly to the in vitro data panels DIV 5 – DIV 9 – DIV 12 under “In Vitro Data from the MEA_NFA Assay”. A 1, B 1, and C 1 show raster plots of the simulated data with increasing levels of synapse formation as detected at 16 electrodes. Parameter values for each run are given in red. A 2 a and A 2 b show all firing of inhibitory and excitatory electrodes – not just the firing as detected at electrodes. Same for B 2 a, B 2 b, C 2 a, C 2 b. These simulation results suggest that bursting occurs when the firing of inhibitory neurons is completely, or almost completely inhibited. Under this condition, and with the excitatory neurons being in a highly connected (synapsed) network, bursting is more likely to occur. Future Directions Though preliminary, these results suggest that the MEA model can provide mechanistic insight into neuron firing data as it evolves over DIV 1 – 12. The results shown above illustrate simulated changes in firing behavior associated with changes in the extent of synapse formation and in the fraction of neurons touching electrodes. Future efforts will include model refinement (incorporating the explicit spatial distribution of the electrodes) and analysis of in vitro data where the MEA neuronal cultures are treated with test compounds.