Enzymes Review of Reaction Terms DG Free Energy



- Slides: 19

Enzymes

Review of Reaction Terms • DG = (Free Energy of Products) - (Free Energy of Reactants)

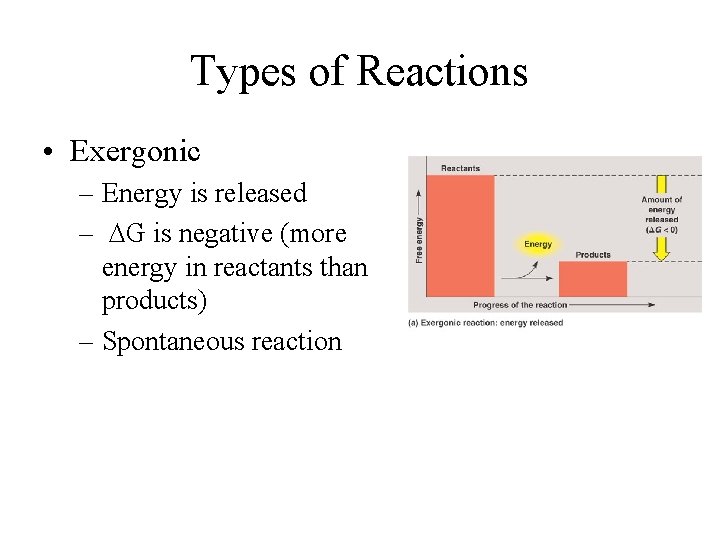
Types of Reactions • Exergonic – Energy is released – DG is negative (more energy in reactants than products) – Spontaneous reaction

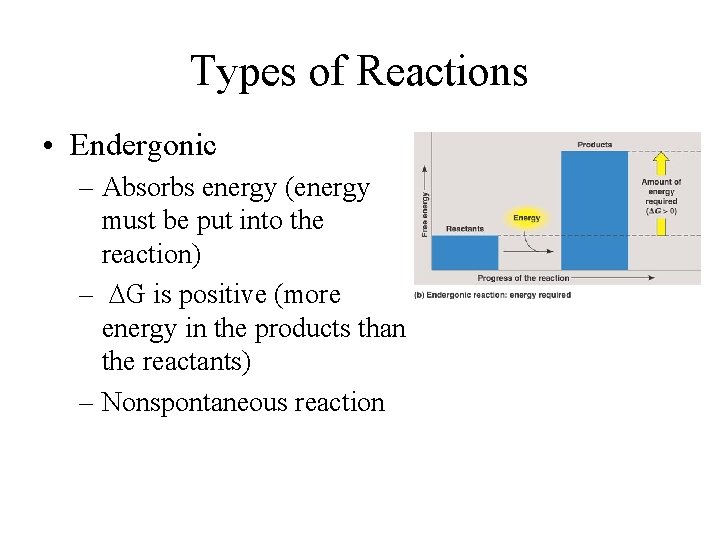
Types of Reactions • Endergonic – Absorbs energy (energy must be put into the reaction) – DG is positive (more energy in the products than the reactants) – Nonspontaneous reaction

Enzyme Function • Enzymes are proteins that speed up reactions – Typically end in “ase” (ex. lactase) • Highly specific – Substrate = the molecules an enzyme acts on • Can be regulated • Return to original structure/shape after reaction – reusable

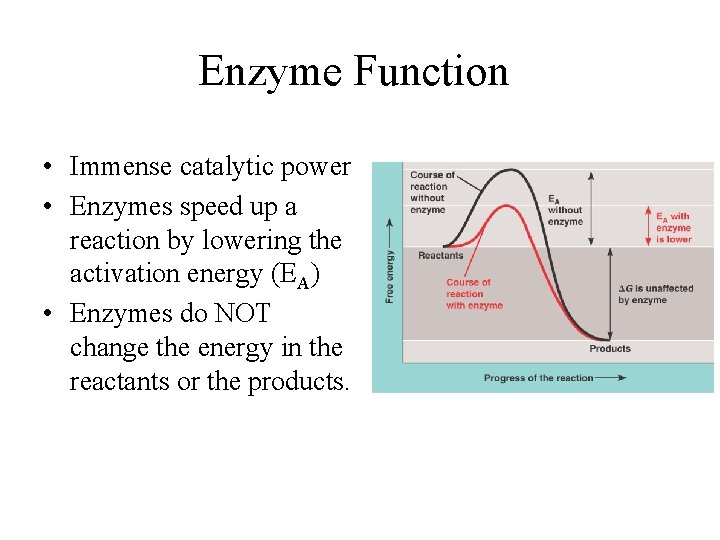
Enzyme Function • Immense catalytic power • Enzymes speed up a reaction by lowering the activation energy (EA) • Enzymes do NOT change the energy in the reactants or the products.

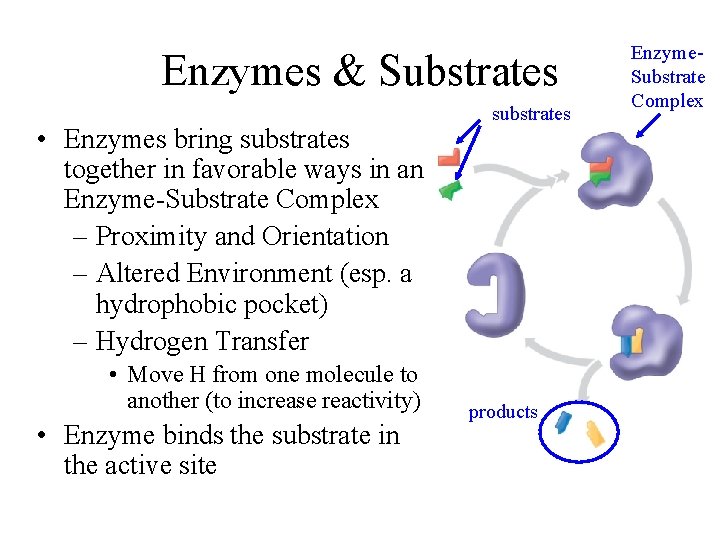
Enzymes & Substrates • Enzymes bring substrates together in favorable ways in an Enzyme-Substrate Complex – Proximity and Orientation – Altered Environment (esp. a hydrophobic pocket) – Hydrogen Transfer • Move H from one molecule to another (to increase reactivity) • Enzyme binds the substrate in the active site substrates products Enzyme. Substrate Complex

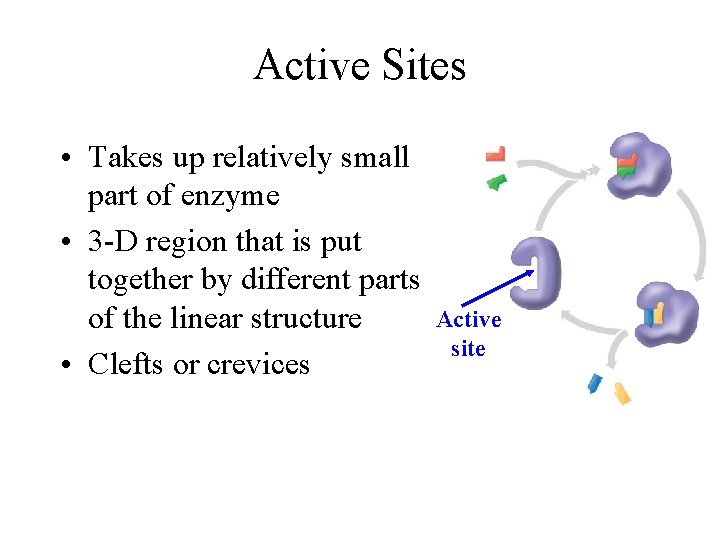
Active Sites • Takes up relatively small part of enzyme • 3 -D region that is put together by different parts of the linear structure • Clefts or crevices Active site

Active Sites • Substrate bound to active site by: – Ionic attractions – Van der waals forces – Hydrophobic interactions – Hydrogen bonds

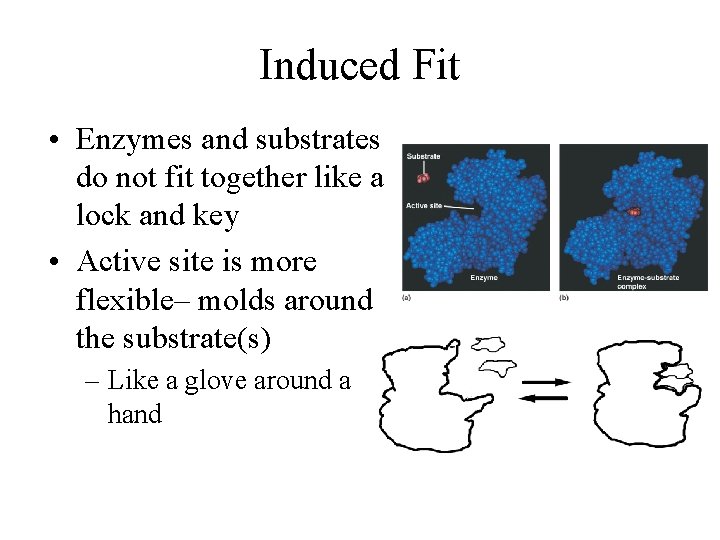
Induced Fit • Enzymes and substrates do not fit together like a lock and key • Active site is more flexible– molds around the substrate(s) – Like a glove around a hand

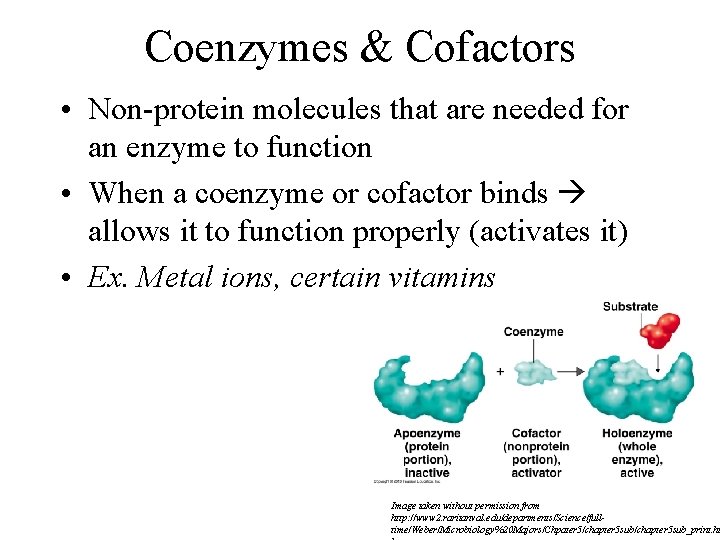
Coenzymes & Cofactors • Non-protein molecules that are needed for an enzyme to function • When a coenzyme or cofactor binds allows it to function properly (activates it) • Ex. Metal ions, certain vitamins Image taken without permission from http: //www 2. raritanval. edu/departments/Science/fulltime/Weber/Microbiology%20 Majors/Chpater 5/chapter 5 sub_print. ht

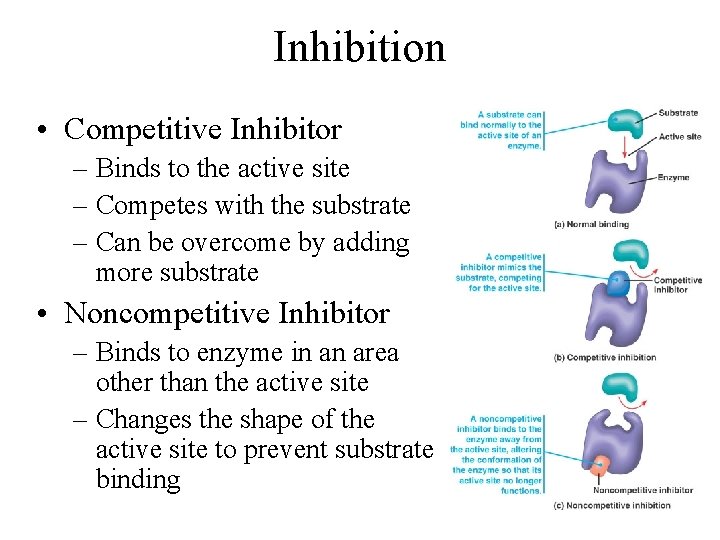
Inhibition • Competitive Inhibitor – Binds to the active site – Competes with the substrate – Can be overcome by adding more substrate • Noncompetitive Inhibitor – Binds to enzyme in an area other than the active site – Changes the shape of the active site to prevent substrate binding

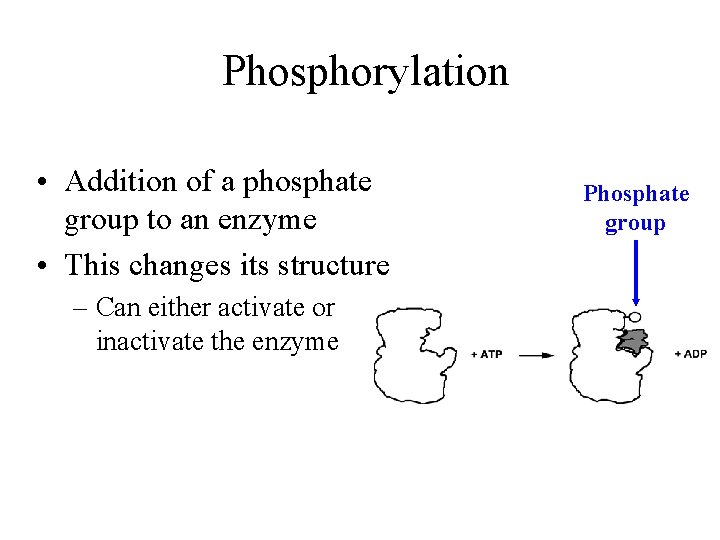
Phosphorylation • Addition of a phosphate group to an enzyme • This changes its structure – Can either activate or inactivate the enzyme Phosphate group

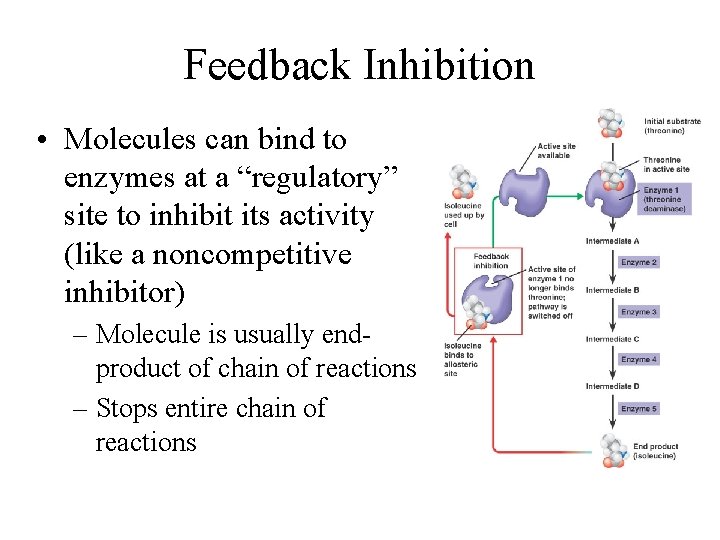
Feedback Inhibition • Molecules can bind to enzymes at a “regulatory” site to inhibit its activity (like a noncompetitive inhibitor) – Molecule is usually endproduct of chain of reactions – Stops entire chain of reactions

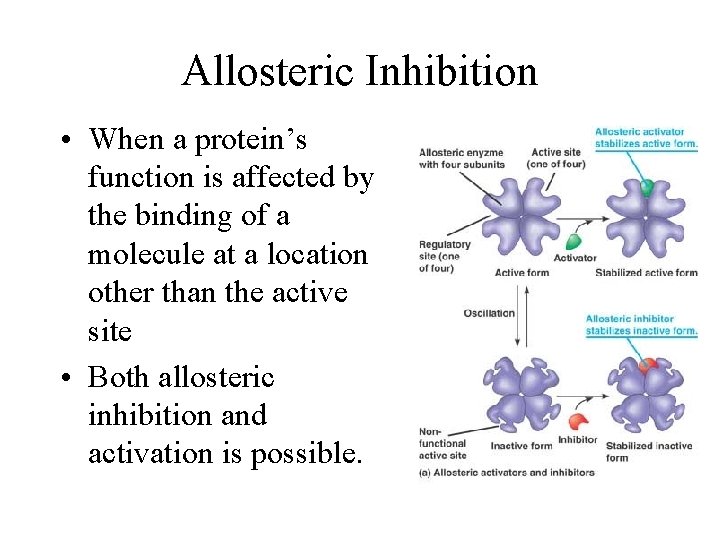
Allosteric Inhibition • When a protein’s function is affected by the binding of a molecule at a location other than the active site • Both allosteric inhibition and activation is possible.

Cooperativity • Occurs in enzymes with multiple subunits (chains) • Binding a substrate to one subunit makes it easier for all of the rest of the subunits to bind substrates

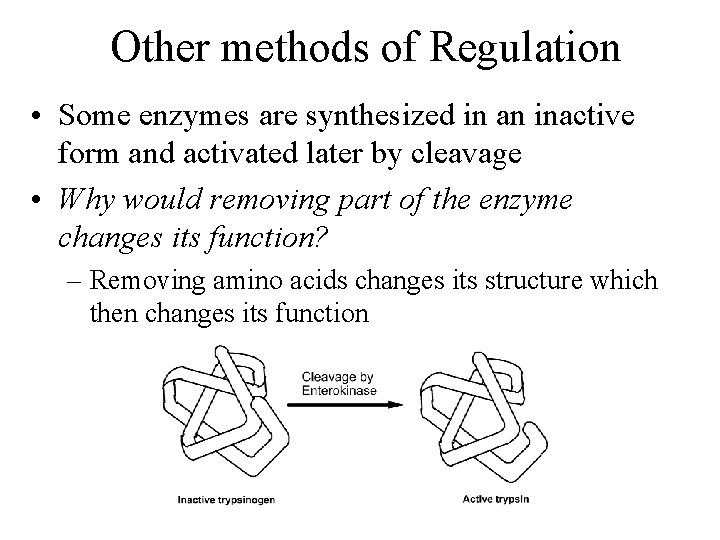
Other methods of Regulation • Some enzymes are synthesized in an inactive form and activated later by cleavage • Why would removing part of the enzyme changes its function? – Removing amino acids changes its structure which then changes its function

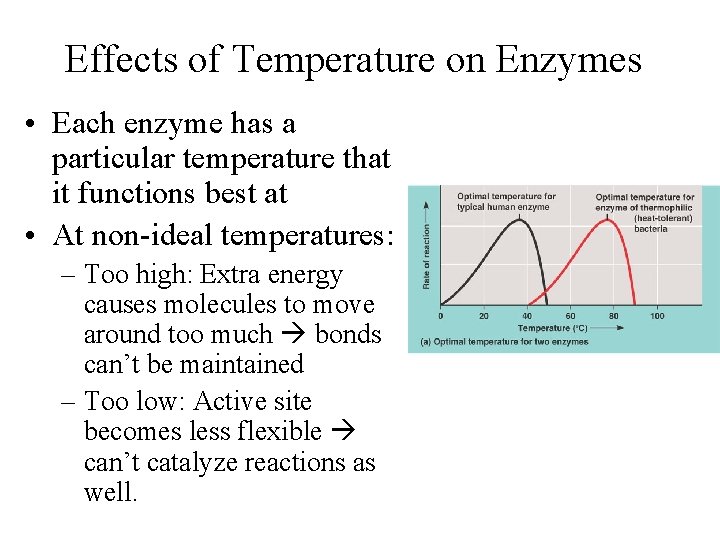
Effects of Temperature on Enzymes • Each enzyme has a particular temperature that it functions best at • At non-ideal temperatures: – Too high: Extra energy causes molecules to move around too much bonds can’t be maintained – Too low: Active site becomes less flexible can’t catalyze reactions as well.

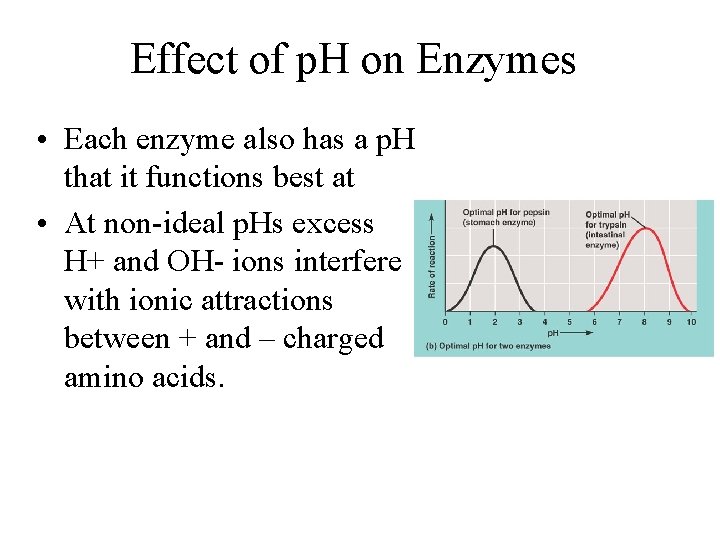
Effect of p. H on Enzymes • Each enzyme also has a p. H that it functions best at • At non-ideal p. Hs excess H+ and OH- ions interfere with ionic attractions between + and – charged amino acids.