ENZYMES Reactants and Products Reactants raw materials that








- Slides: 23

ENZYMES

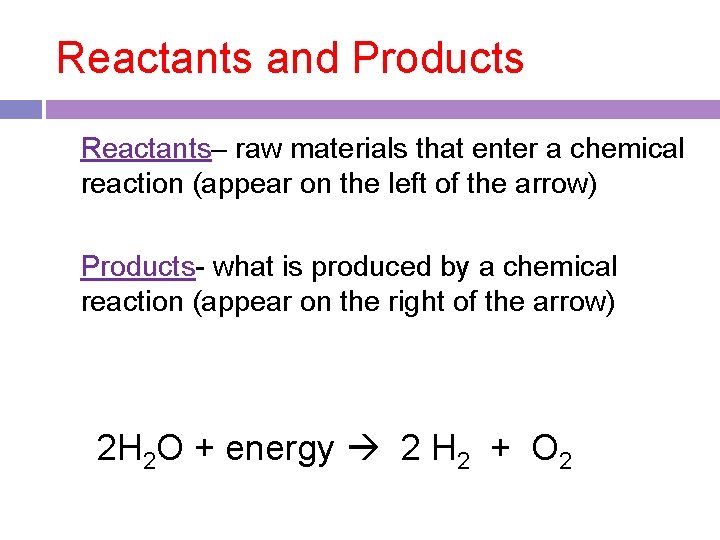
Reactants and Products Reactants– raw materials that enter a chemical reaction (appear on the left of the arrow) Products- what is produced by a chemical reaction (appear on the right of the arrow) 2 H 2 O + energy 2 H 2 + O 2

Energy Changes -Energy is released or absorbed whenever chemical bonds are broken or formed during chemical reactions.

Energy Sources -Every organism must have a source of energy to carry out the chemical reactions it needs to stay alive. -Humans release the energy needed to grow, breathe, think, and even dream through the chemical reactions that occur when we metabolize, or break down, digested food.

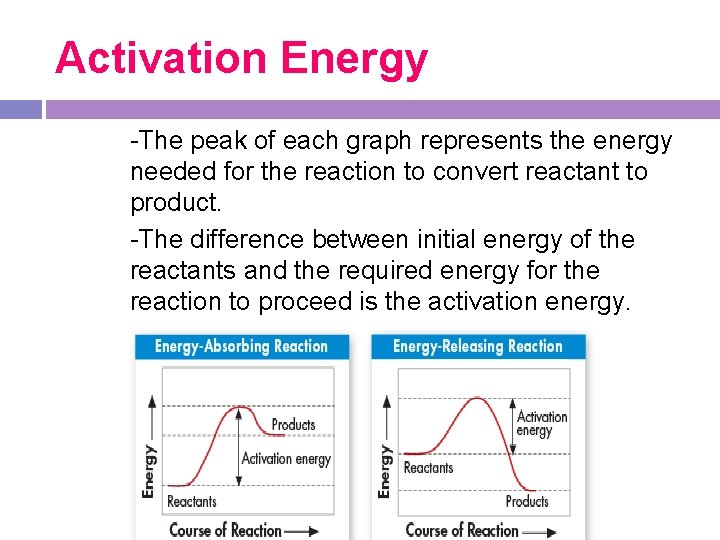
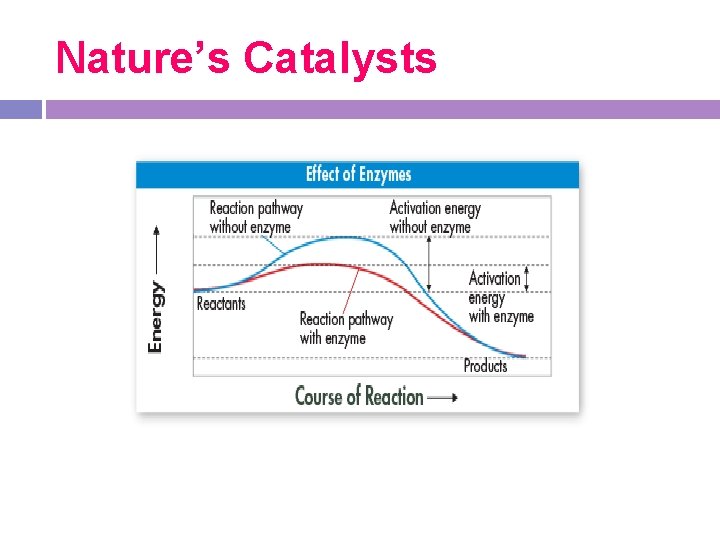
Activation Energy All chemical reactions that release energy do not occur spontaneously. The energy that is needed to get a reaction started is called the activation energy. -

Activation Energy -The peak of each graph represents the energy needed for the reaction to convert reactant to product. -The difference between initial energy of the reactants and the required energy for the reaction to proceed is the activation energy.

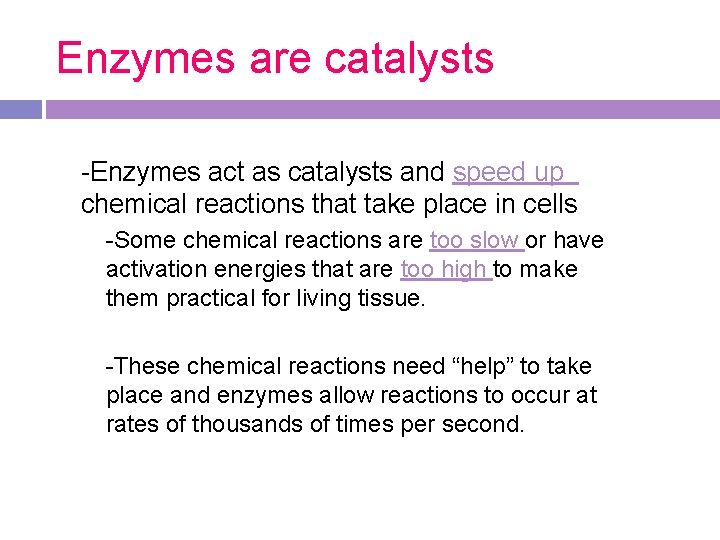
Catalysts -A catalyst is a substance that speeds up the rate of a chemical reaction. -Catalysts work by lowering a reaction’s activation energy. -Without catalysts to lower the activation energy, many reactions cannot proceed.

enzymes -substances that start or speed up a biological chemical reaction by lowering the activation energy

Enzymes are catalysts -Enzymes act as catalysts and speed up chemical reactions that take place in cells -Some chemical reactions are too slow or have activation energies that are too high to make them practical for living tissue. -These chemical reactions need “help” to take place and enzymes allow reactions to occur at rates of thousands of times per second.

Enzyme Characteristics -Enzymes are very specific, generally catalyzing only one chemical reaction. -Most biological enzymes are proteins -They are reusable -They usually end with -ase -Part of an enzyme’s name is usually derived from the reaction it catalyzes.

Specificity in Enzymes Ex> sucrose, a disacharide, must be digested to monosaccharides so cells can absorb it; the enzyme sucrase is required; sucrase is specific to sucrose, it will not affect other disaccharides such as lactose.

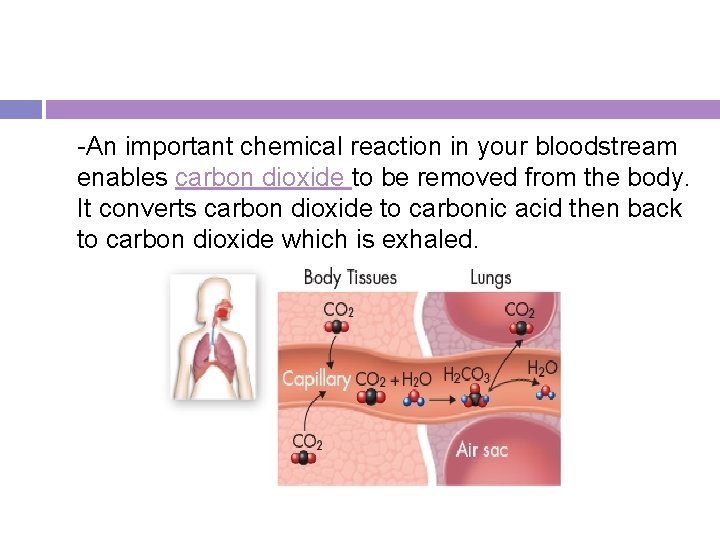
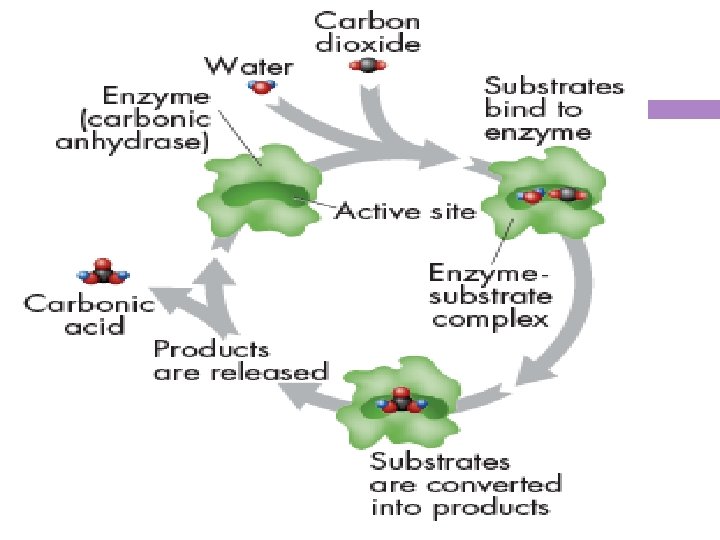
-An important chemical reaction in your bloodstream enables carbon dioxide to be removed from the body. It converts carbon dioxide to carbonic acid then back to carbon dioxide which is exhaled.

Nature’s Catalysts -This reaction happens so slowly that carbon dioxide might build up in the body faster than the bloodstream could remove it. -Your bloodstream contains an enzyme called carbonic anhydrase that speeds up the reaction by a factor of 10 million, so that the reaction takes place immediately and carbon dioxide can be removed from the blood quickly.

Nature’s Catalysts �

substrates -the reactants of enzyme-catalyzed reactions; the substance that enzymes act on

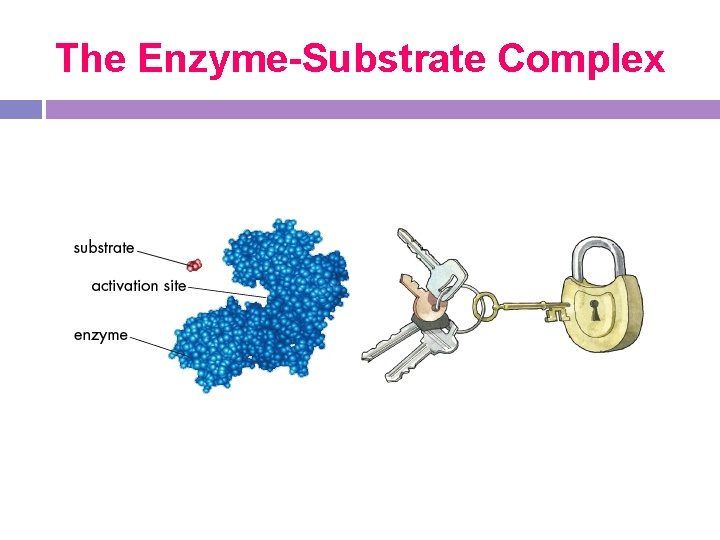
Active site -place on the enzyme where substrate binds -the active site and the substrates have complementary shapes, each specific for its substrate


Lock and Key Model -Enzymes have a “lock & key” fit with their active sites & substrates

The Enzyme-Substrate Complex

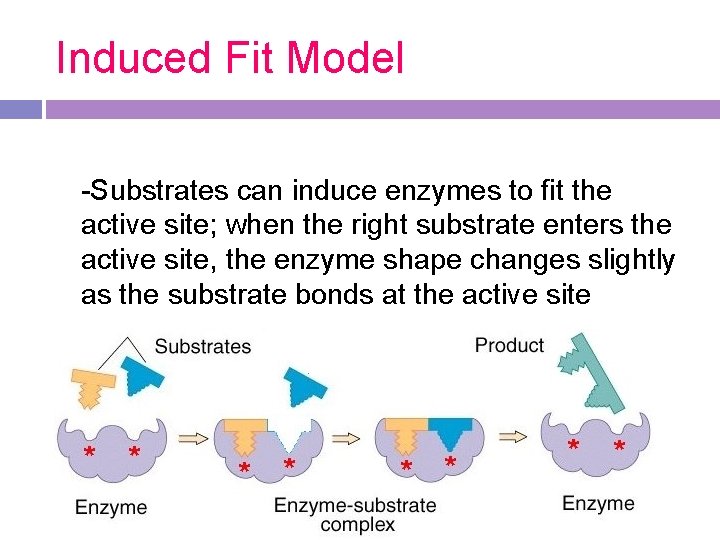
Induced Fit Model -Substrates can induce enzymes to fit the active site; when the right substrate enters the active site, the enzyme shape changes slightly as the substrate bonds at the active site

Factors affecting enzyme activity 1. temperature 2. p. H

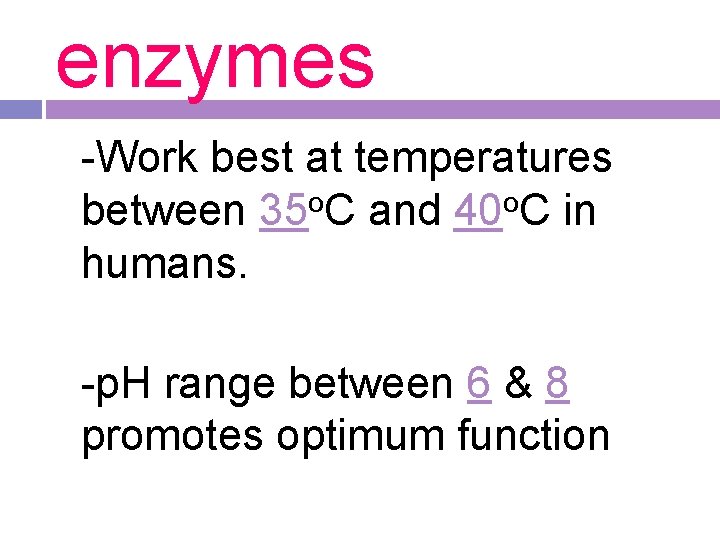
enzymes -Work best at temperatures o o between 35 C and 40 C in humans. -p. H range between 6 & 8 promotes optimum function

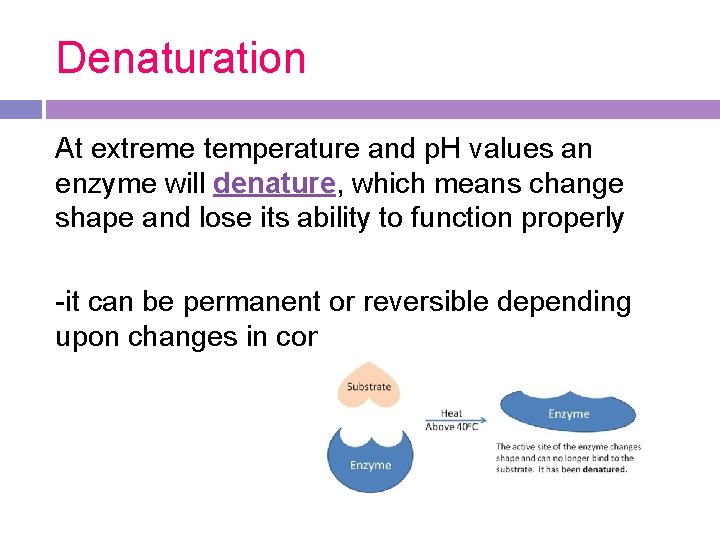
Denaturation At extreme temperature and p. H values an enzyme will denature, which means change shape and lose its ability to function properly -it can be permanent or reversible depending upon changes in conditions