Enzymes Metabolism Chapter 8 I Energy Review A

Enzymes & Metabolism Chapter 8
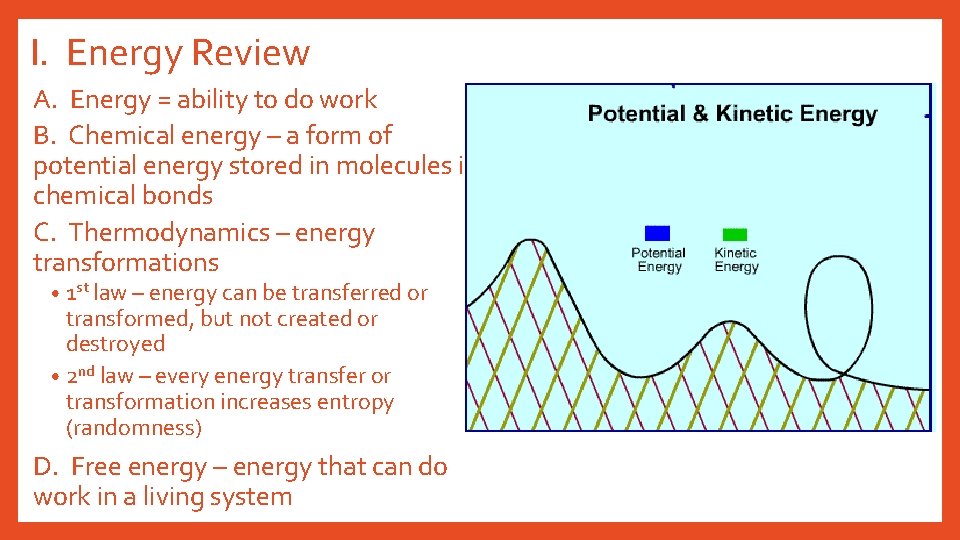
I. Energy Review A. Energy = ability to do work B. Chemical energy – a form of potential energy stored in molecules in chemical bonds C. Thermodynamics – energy transformations • 1 st law – energy can be transferred or transformed, but not created or destroyed • 2 nd law – every energy transfer or transformation increases entropy (randomness) D. Free energy – energy that can do work in a living system
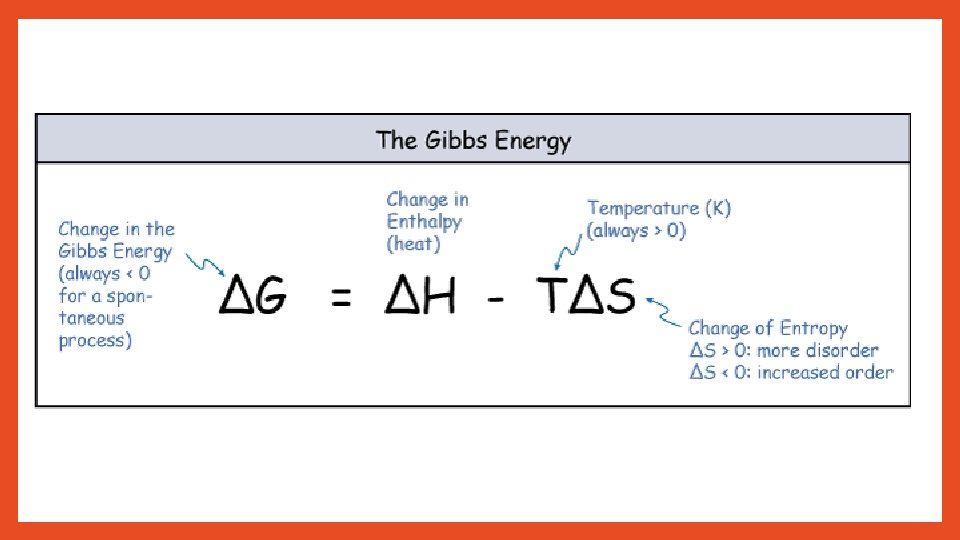
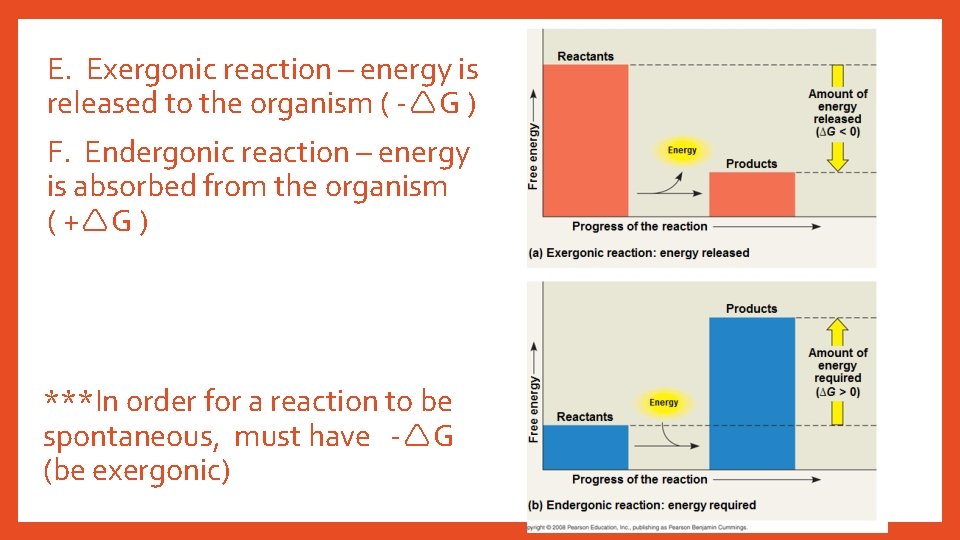
E. Exergonic reaction – energy is released to the organism ( - G ) F. Endergonic reaction – energy is absorbed from the organism ( + G ) ***In order for a reaction to be spontaneous, must have - G (be exergonic)
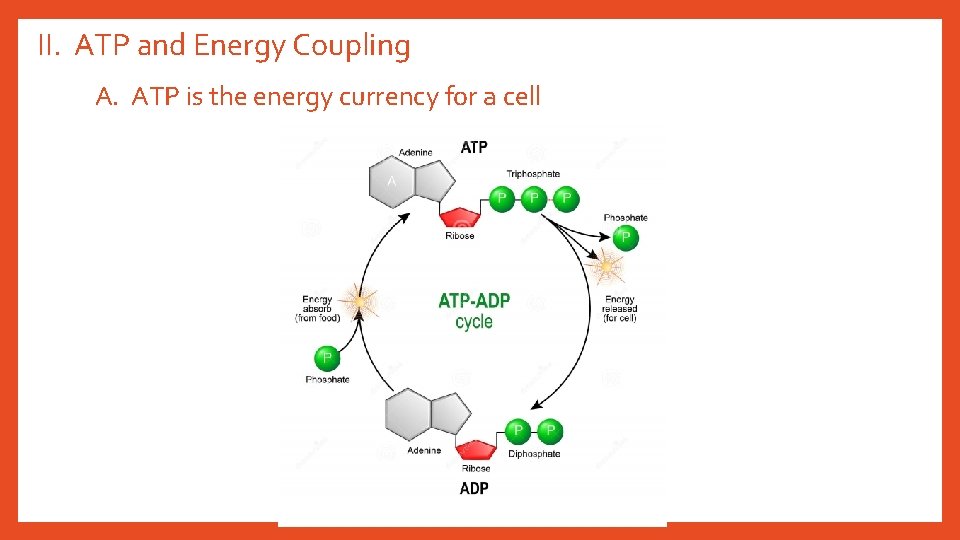
II. ATP and Energy Coupling A. ATP is the energy currency for a cell
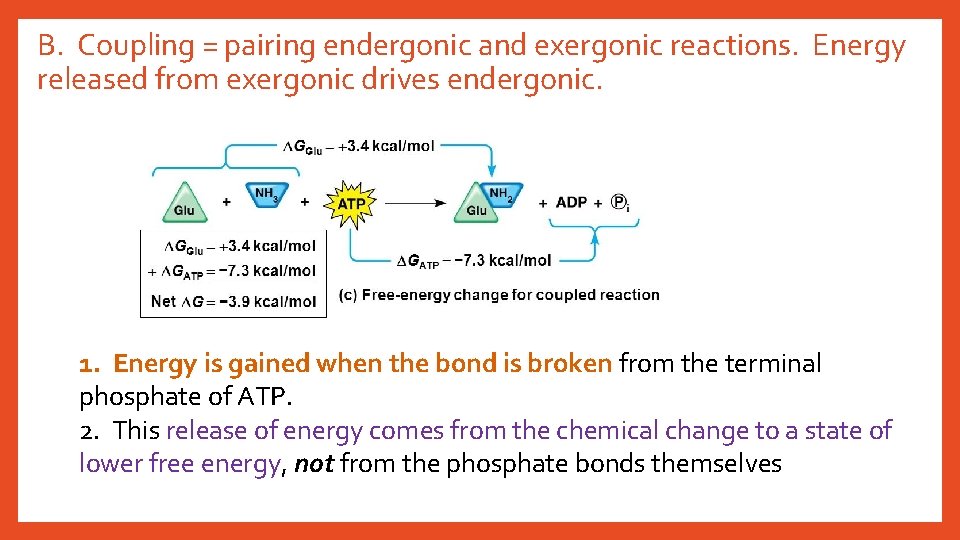
B. Coupling = pairing endergonic and exergonic reactions. Energy released from exergonic drives endergonic. 1. Energy is gained when the bond is broken from the terminal phosphate of ATP. 2. This release of energy comes from the chemical change to a state of lower free energy, not from the phosphate bonds themselves
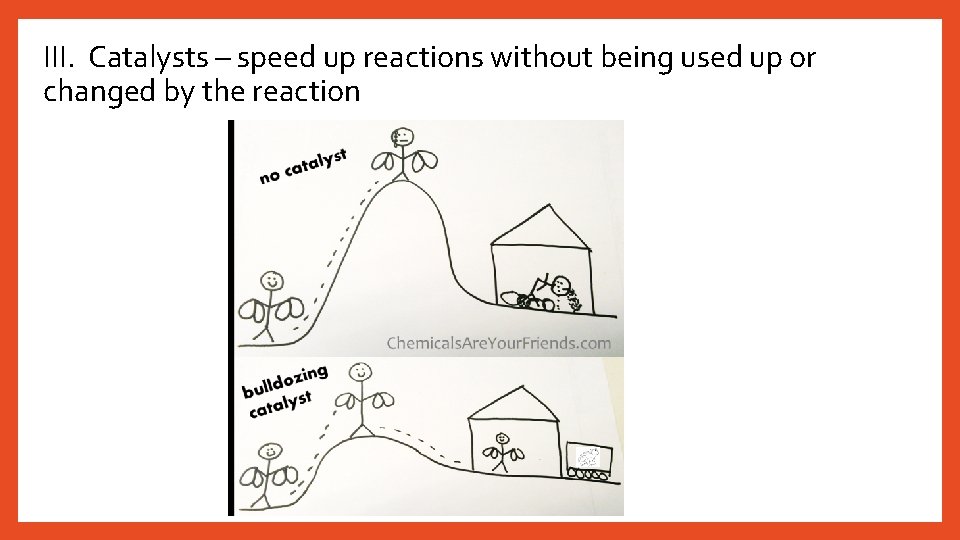
III. Catalysts – speed up reactions without being used up or changed by the reaction
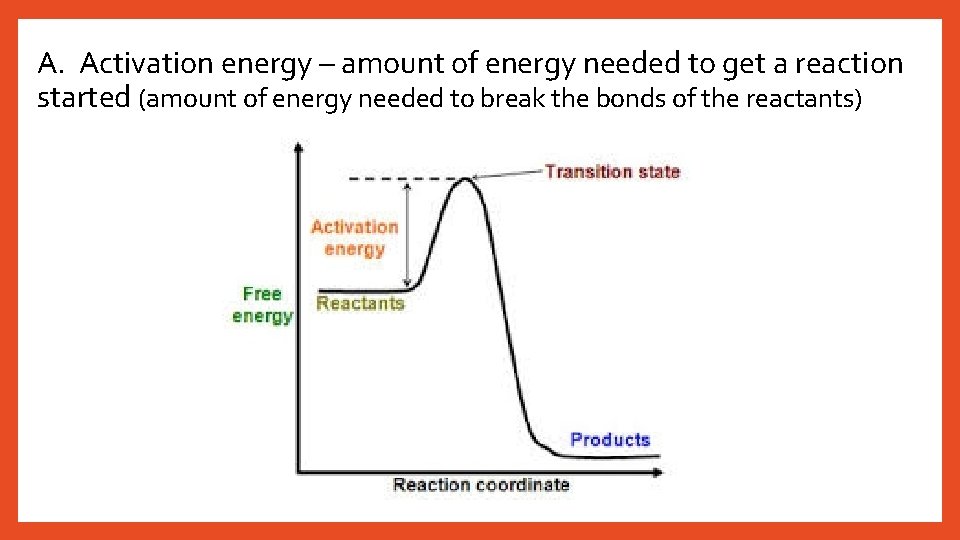
A. Activation energy – amount of energy needed to get a reaction started (amount of energy needed to break the bonds of the reactants)
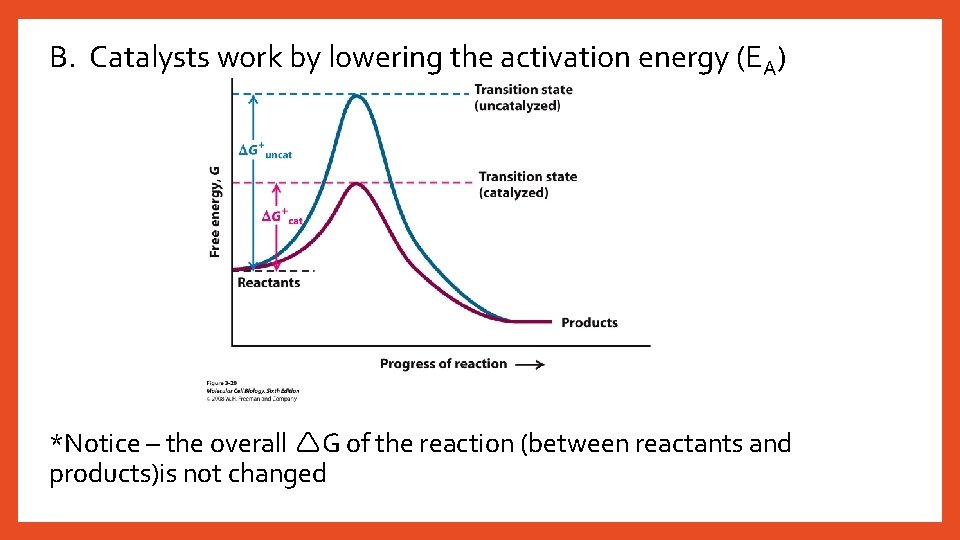
B. Catalysts work by lowering the activation energy (EA) *Notice – the overall G of the reaction (between reactants and products)is not changed
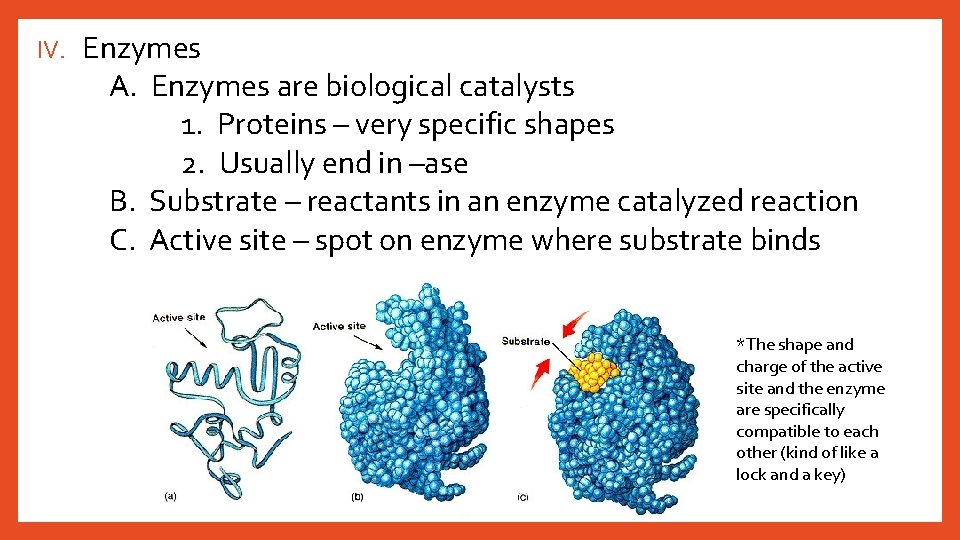
IV. Enzymes A. Enzymes are biological catalysts 1. Proteins – very specific shapes 2. Usually end in –ase B. Substrate – reactants in an enzyme catalyzed reaction C. Active site – spot on enzyme where substrate binds *The shape and charge of the active site and the enzyme are specifically compatible to each other (kind of like a lock and a key)
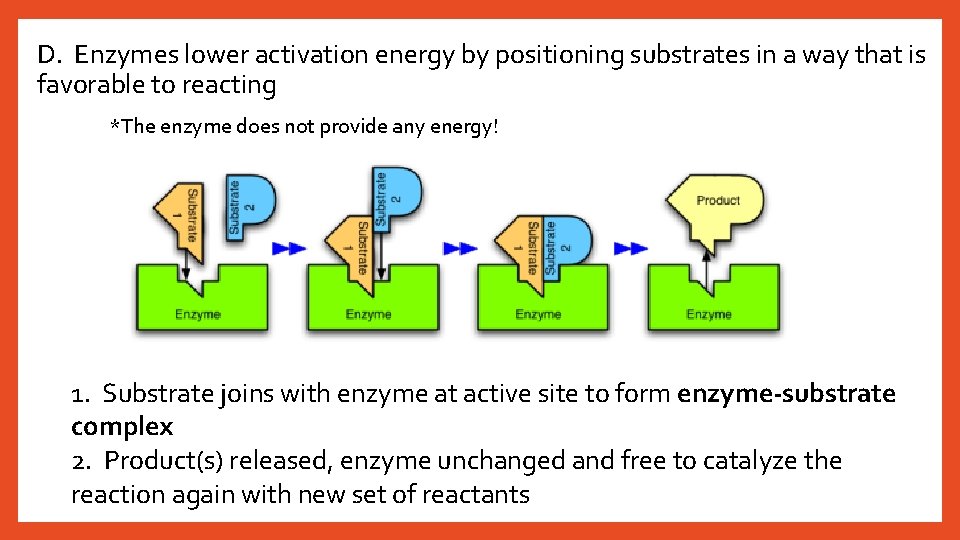
D. Enzymes lower activation energy by positioning substrates in a way that is favorable to reacting *The enzyme does not provide any energy! 1. Substrate joins with enzyme at active site to form enzyme-substrate complex 2. Product(s) released, enzyme unchanged and free to catalyze the reaction again with new set of reactants
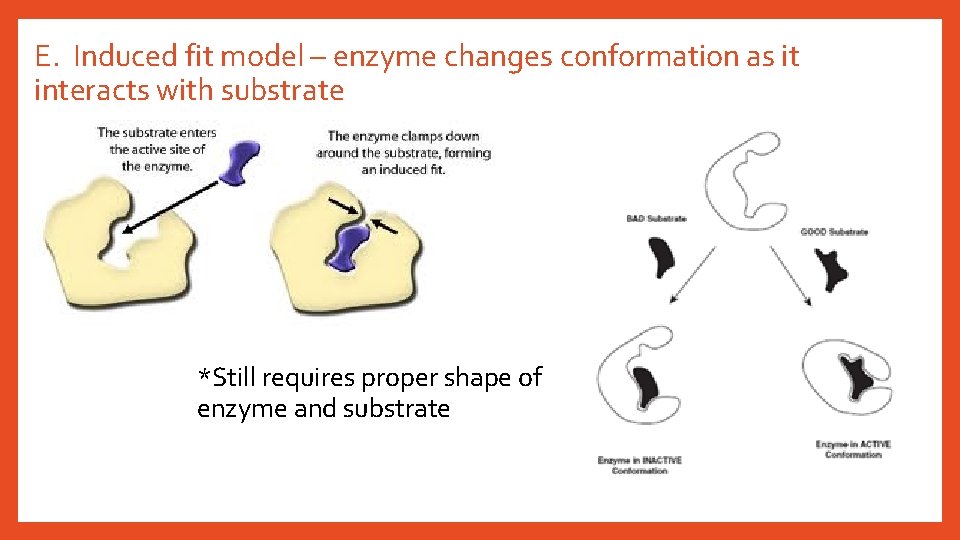
E. Induced fit model – enzyme changes conformation as it interacts with substrate *Still requires proper shape of enzyme and substrate
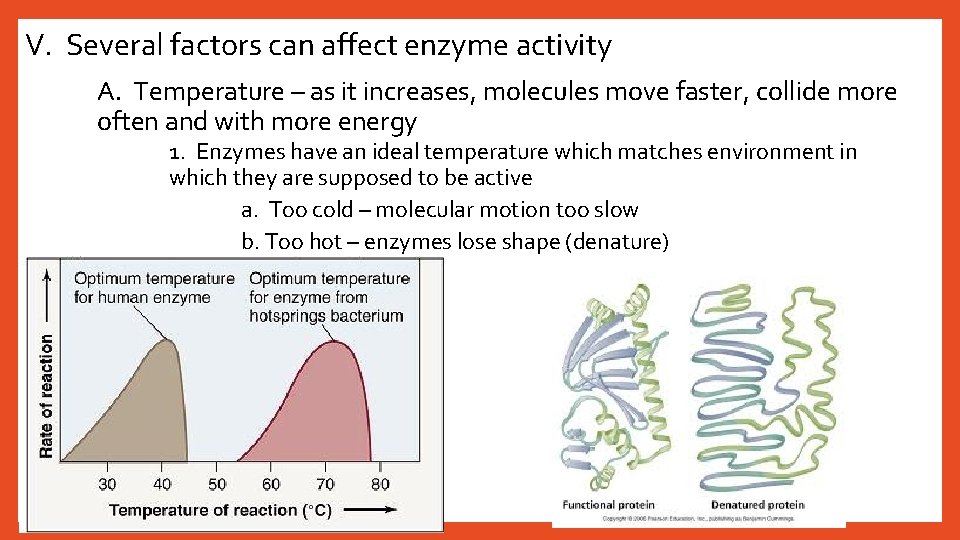
V. Several factors can affect enzyme activity A. Temperature – as it increases, molecules move faster, collide more often and with more energy 1. Enzymes have an ideal temperature which matches environment in which they are supposed to be active a. Too cold – molecular motion too slow b. Too hot – enzymes lose shape (denature)
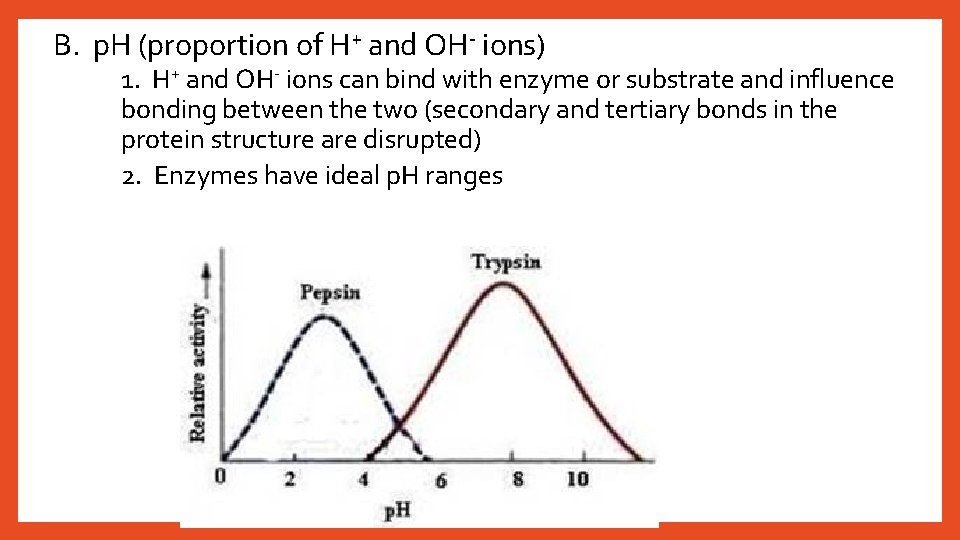
B. p. H (proportion of H+ and OH- ions) 1. H+ and OH- ions can bind with enzyme or substrate and influence bonding between the two (secondary and tertiary bonds in the protein structure are disrupted) 2. Enzymes have ideal p. H ranges

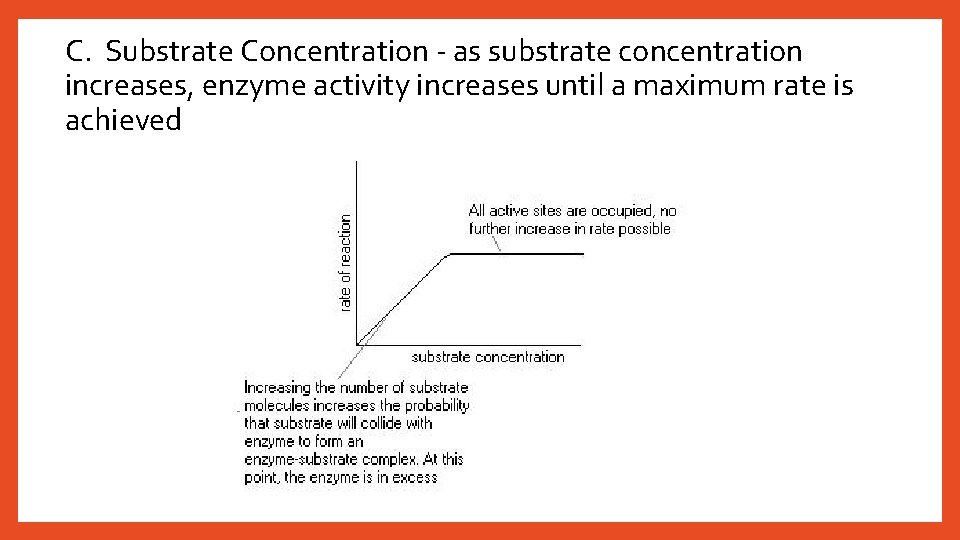
C. Substrate Concentration - as substrate concentration increases, enzyme activity increases until a maximum rate is achieved
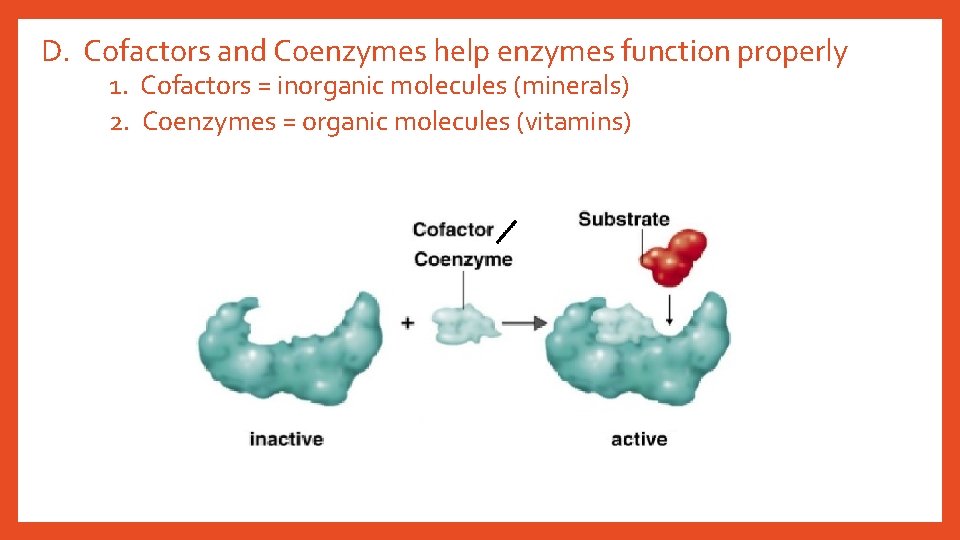
D. Cofactors and Coenzymes help enzymes function properly 1. Cofactors = inorganic molecules (minerals) 2. Coenzymes = organic molecules (vitamins)
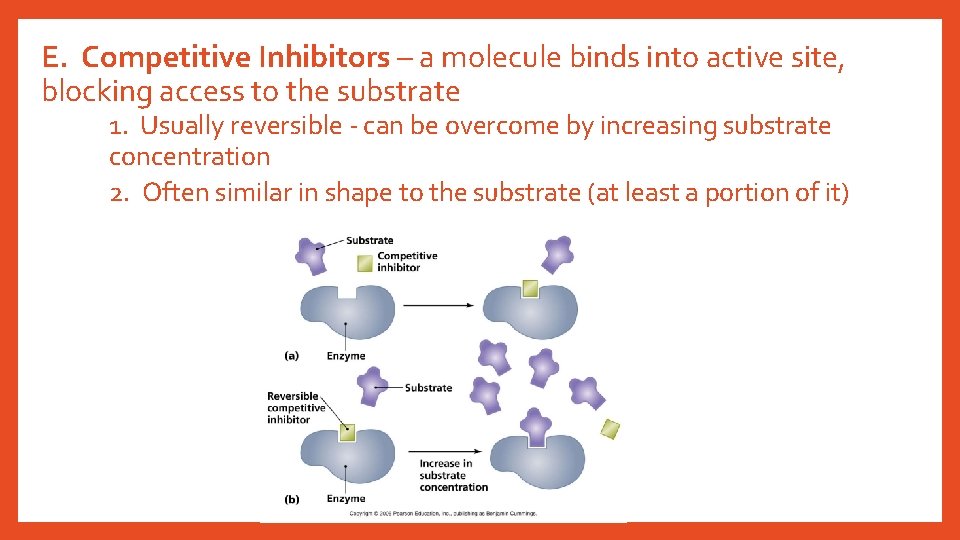
E. Competitive Inhibitors – a molecule binds into active site, blocking access to the substrate 1. Usually reversible - can be overcome by increasing substrate concentration 2. Often similar in shape to the substrate (at least a portion of it)
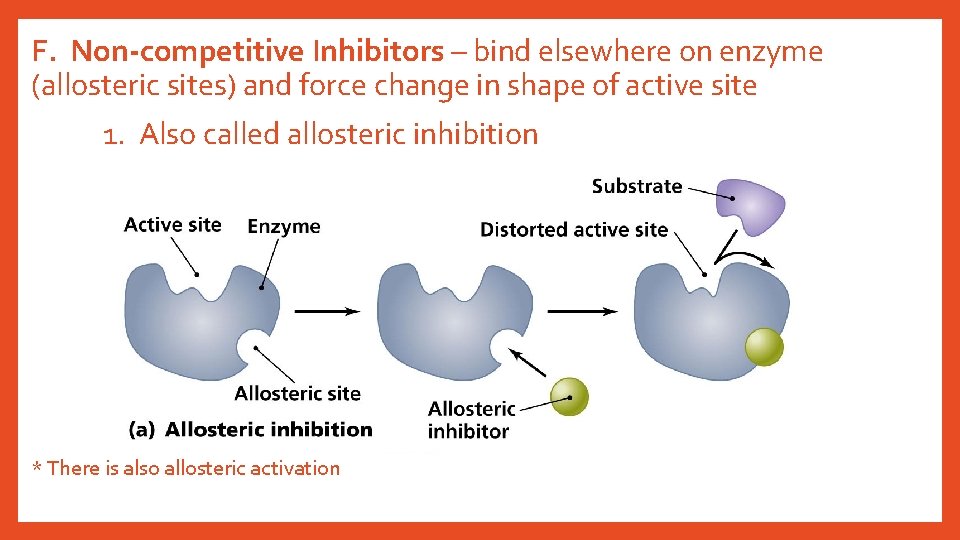
F. Non-competitive Inhibitors – bind elsewhere on enzyme (allosteric sites) and force change in shape of active site 1. Also called allosteric inhibition * There is also allosteric activation
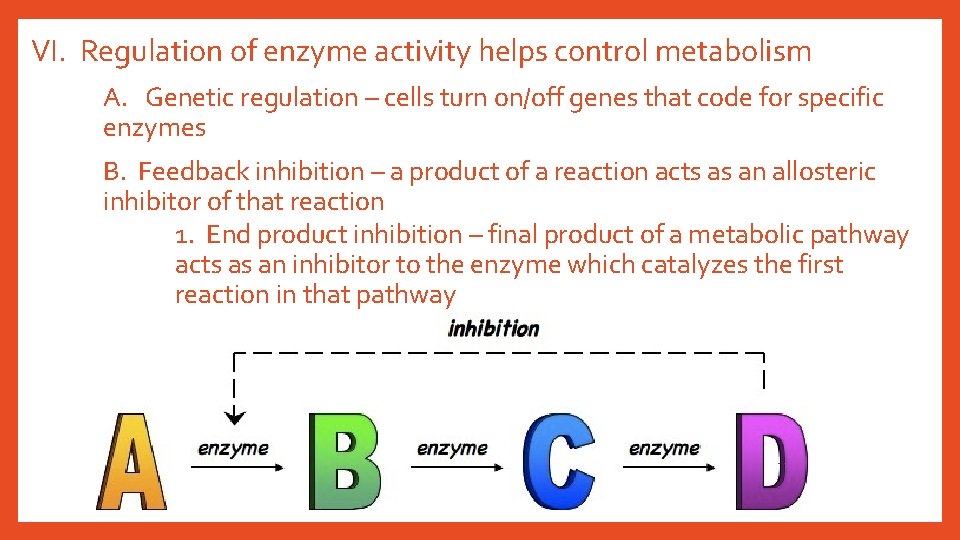
VI. Regulation of enzyme activity helps control metabolism A. Genetic regulation – cells turn on/off genes that code for specific enzymes B. Feedback inhibition – a product of a reaction acts as an allosteric inhibitor of that reaction 1. End product inhibition – final product of a metabolic pathway acts as an inhibitor to the enzyme which catalyzes the first reaction in that pathway
- Slides: 20